Mucus hypersecretion in asthma: causes and effects
- PMID: 19077699
- PMCID: PMC2709596
- DOI: 10.1097/MCP.0b013e32831da8d3
Mucus hypersecretion in asthma: causes and effects
Abstract
Purpose of review: Airway mucus plugging has long been recognized as a principal cause of death in asthma. However, molecular mechanisms of mucin overproduction and secretion have not been understood until recently. These mechanisms are reviewed together with ongoing investigations relating them to lung pathophysiology.
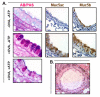
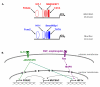
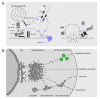
Recent findings: Of the five secreted gel-forming mucins in mammals, only MUC5AC and MUC5B are produced in significant quantities in intrapulmonary airways. MUC5B is the principal gel-forming mucin at baseline in small airways of humans and mice, and therefore likely performs most homeostatic clearance functions. MUC5AC is the principal gel-forming mucin upregulated in airway inflammation and is under negative control by forkhead box a2 (Foxa2) and positive control by hypoxia inducible factor-1 (HIF-1). Mucin secretion is regulated separately from production, principally by extracellular triphosphate nucleotides that bind P2Y2 receptors on the lumenal surface of airway secretory cells, generating intracellular second messengers that activate the exocytic proteins, Munc13-2 and synaptotagmin-2.
Summary: Markedly upregulated production of MUC5AC together with stimulated secretion leads to airflow obstruction in asthma. As MUC5B appears to mediate homeostatic functions, it may be possible to selectively inhibit MUC5AC production without impairing airway function. The precise roles of mucin hypersecretion in asthma symptoms such as dyspnea and cough and in physiologic phenomena such as airway hyperresponsiveness remain to be defined.
Figures
References
-
- Thornton DJ, Sheehan JK. From mucins to mucus: toward a more coherent understanding of this essential barrier. Proc Am Thorac Soc. 2004;1:54–61. - PubMed
-
-
Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol. 2008;70:459–486. An authoritative, up-to-date review of the gel-forming mucins of the airway.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

