Cholera toxin, E. coli heat-labile toxin, and non-toxic derivatives induce dendritic cell migration into the follicle-associated epithelium of Peyer's patches
- PMID: 19079161
- PMCID: PMC2614317
- DOI: 10.1038/mi.2007.7
Cholera toxin, E. coli heat-labile toxin, and non-toxic derivatives induce dendritic cell migration into the follicle-associated epithelium of Peyer's patches
Abstract
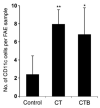
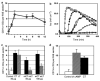
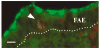
The follicle-associated epithelium (FAE) of Peyer's patches (PPs) transports antigens and microorganisms into mucosal lymphoid tissues where they are captured by subepithelial dendritic cells (DCs). Feeding of cholera toxin (CT) induced migration of subepithelial DCs to interfollicular T-cell areas within 24 h. This study investigated short-term effects of CT, Escherichia coli heat-labile toxin, and non-toxic derivatives on DC migration. CT or CTB injected into ligated intestinal loops induced significant increase in CD11c+ DCs within the FAE within 90 min. In mice fed CT intragastrically, DC numbers in the FAE increased by 1 h, were maximal by 2 h, declined between 8 and 12 h, and were reversed by 24 h. Feeding of native LT, recombinant CTB, dibutyryl cyclic AMP, and to a lesser extent mutated CT(E29H) or mutated LT(R192G) had the same effect. Thus, both A and B subunits of enterotoxins, presumably acting through distinct signaling pathways, may promote capture of incoming antigens and pathogens by PP DCs.
Conflict of interest statement
Figures


References
-
- Strober W, McGhee JR. Inductive and effector tissues and cells of the mucosal immune system. In: Mestecky J, Lamm M, McGhee J, Bienenstock J, Mayer L, Strober W, editors. Mucosal Immunology. Academic Press; New York: 2005. pp. 371–374.
-
- Neutra MR, Mantis NJ, Kraehenbuhl JP. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat Immunol. 2001;2:1004–1009. - PubMed
-
- Cook DN, Prosser DM, Forster R, Zhang J, Kuklin NA, Abbondanzo SJ, et al. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 2000;12:495–503. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

