Distal IgA immunity can be sustained by alphaEbeta7+ B cells in L-selectin-/- mice following oral immunization
- PMID: 19079162
- PMCID: PMC9811399
- DOI: 10.1038/mi.2007.2
Distal IgA immunity can be sustained by alphaEbeta7+ B cells in L-selectin-/- mice following oral immunization
Abstract
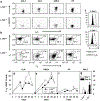
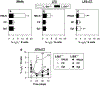
Understanding the role of homing receptors could aid vaccine strategies for developing distal mucosal immunity. Infection studies have revealed that immune intestinal B cells use alpha(4)beta(7) homing receptors, but their role in subsequent oral immunization with soluble antigens is unknown. To assess the influence of L-selectin and alpha(4)beta(7) on distal B cells following oral cholera toxin (CT) immunization, L-selectin-deficient (L-Sel(-/-)) IgA anti-CT-B-specific B cells were enhanced 30-, 9.2-, and 3.5-fold in head and neck lymph nodes (HNLNs), nasal-associated lymphoid tissue, and nasal passages (NPs), respectively, vs. L-Sel(+/+) mice. Cell-sorted intestinal and NP IgA antibody-forming cells (AFCs) were mostly alpha(4)beta(7)(+), unlike HNLN L-Sel(-/-) IgA and IgG anti-CT-B AFCs that were alpha(E)beta(7)(+), contrasting with L-Sel(+/+) HNLN IgA AFCs that were mostly alpha(4)beta(7)(+). In vitro studies revealed that L-Sel(-/-) HNLN B cells preferentially expressed alpha(E) following polyclonal stimulation. These studies show that HNLN B cells express alpha(E)beta(7) in the absence of L-selectin to sustain distal IgA responses.
Conflict of interest statement
DISCLOSURE
The authors declared no conflict of interest.
Figures






References
-
- Rudzik R, Clancy RL, Perey DY, Day RP & Bienenstock J Repopulation with IgA-containing cells of bronchial and intestinal lamina propria after transfer of homologous Peyer’s patch and bronchial lymphocytes. J. Immunol 114, 1599–1604 (1975). - PubMed
-
- Mestecky J, McGhee JR, Michalek SM, Arnold RR, Crago SS & Babb JL Concept of the local and common mucosal immune response. Adv. Exp. Med. Biol 107, 185–192 (1978). - PubMed
-
- McDermott MR & Bienenstock J Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J. Immunol 122, 1892–1898 (1979). - PubMed
-
- Quiding-Jarbrink M, Nordstrom L, Granstrom G, Kilander A, Jertbom M, Butcher EC et al. Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric, and nasal immunizations. A molecular basis for the compartmentalization of effector B cell responses. J. Clin. Invest 99, 1281–1286 (1997). - PMC - PubMed
-
- Kantele A, Hakkinen M, Moldoveanu Z, Lu A, Savilahti E, Alvarez RD et al. Differences in immune responses induced by oral and rectal immunizations with Salmonella typhi Ty21a: evidence for compartmentalization within the common mucosal immune system in humans. Infect. Immun 66, 5630–5635 (1998). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

