TREM-2 binds to lipooligosaccharides of Neisseria gonorrhoeae and is expressed on reproductive tract epithelial cells
- PMID: 19079182
- PMCID: PMC2614303
- DOI: 10.1038/mi.2008.1
TREM-2 binds to lipooligosaccharides of Neisseria gonorrhoeae and is expressed on reproductive tract epithelial cells
Abstract
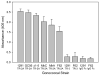
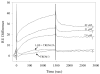
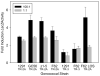
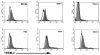
Triggering receptor expressed on myeloid cells-2 (TREM-2) is an innate immune receptor that initiates cellular activation upon ligation. In this study, we examined the interaction of TREM-2 with Neisseria gonorrhoeae using murine TREM-2A, as it has been reported to recognize bacterial ligands. Using a whole-bacteria enzyme-linked immunosorbent assay (ELISA), TREM-2A bound to all six strains in variable degrees. Far-western blots of gonococcal outer membranes revealed TREM-2A binding to lipooligosaccharide (LOS) and opacity (Opa) protein, with predominant binding to LOS. Binding of TREM-2A to LOS was confirmed by ELISA and surface plasmon resonance. O-deacylation of the lipid A significantly reduced binding. Flow cytometry and reporter cell assays showed that gonococci bound to TREM-2A-transfected cells and induced transmembrane signaling. In humans, TREM-2 was constitutively expressed by genitourinary and fallopian tube epithelial cells, both of which are primary targets of gonococcal invasion. Ligation of TREM-2 by LOS induced interleukin-6 production in HeLa cervical carcinoma cells. To our knowledge, this is the first report of the expression of human TREM-2 by cells deriving from a non-myeloid lineage. We conclude that gonococci can interact with TREM-2 receptors through binding to LOS and Opa protein and initiate cell signaling and cytokine production.
Figures





References
-
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance. U S Department of Health and Human Services, Public Health Service. 2005
-
- Paavonen J. Pelvic inflammatory disease. From diagnosis to prevention. Dermatol Clin. 1998;16:747–756. - PubMed
-
- Cohen MS, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868–1873. - PubMed
-
- Chesson HW, Pinkerton SD. Sexually transmitted diseases and the increased risk for HIV transmission: implications for cost-effectiveness analyses of sexually transmitted disease prevention interventions. J Acquir Immune Defic Syndr. 2000;24:48–56. - PubMed
-
- Mabey D. Interactions between HIV infection and other sexually transmitted diseases. Trop Med Int Health. 2000;5:A32–A36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

