Secreted Frizzled-related protein 2 is a procollagen C proteinase enhancer with a role in fibrosis associated with myocardial infarction
- PMID: 19079247
- PMCID: PMC2722759
- DOI: 10.1038/ncb1811
Secreted Frizzled-related protein 2 is a procollagen C proteinase enhancer with a role in fibrosis associated with myocardial infarction
Abstract
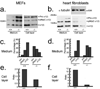
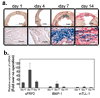
Secreted Frizzled-related proteins (sFRPs) have emerged as key regulators of a wide range of developmental and disease processes. Most of the known functions of mammalian sFRPs have been attributed to their ability to antagonize Wnt signalling. Recently however, Xenopus laevis and zebrafish sFRP, Sizzled, was shown to function as an antagonist of Chordin processing by Tolloid-like metalloproteinases. This has led to the proposal that sFRPs may function as evolutionarily conserved antagonists of chordinase activities of this class of proteinases. In contrast to this proposal, we show here that the mammalian sFRP, sFRP2, does not affect Chordin processing, but instead, can serve as a direct enhancer of procollagen C proteinase activity of Tolloid-like metalloproteinases. We also show that the level of fibrosis, in which procollagen processing by Tolloid-like proteinases has a rate-limiting role, is markedly reduced in Sfrp2-null mice subjected to myocardial infarction. Importantly, this reduced level of fibrosis is accompanied by significantly improved cardiac function. This study thus uncovers a function for sFRP2 and a potential therapeutic application for sFRP2 antagonism in controlling fibrosis in the infarcted heart.
Figures





Comment in
-
sFRPs: a declaration of (Wnt) independence.Nat Cell Biol. 2009 Jan;11(1):13. doi: 10.1038/ncb0109-13. Nat Cell Biol. 2009. PMID: 19122594 No abstract available.
References
-
- Mayr T, et al. Fritz: a secreted frizzled-related protein that inhibits Wnt activity. Mech Dev. 1997;63:109–125. - PubMed
-
- Salic AN, Kroll KL, Evans LM, Kirschner MW. Sizzled: a secreted Xwnt8 antagonist expressed in the ventral marginal zone of Xenopus embryos. Development. 1997;124:4739–4748. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

