Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus
- PMID: 19079257
- PMCID: PMC2615794
- DOI: 10.1038/nm.1892
Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus
Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease mediated by autoantibodies and preferentially affecting women of childbearing age. Because the offspring of mothers with SLE show a high frequency of learning disorders, we hypothesized that maternally transferred autoantibodies that bind DNA and the N-methyl-D-aspartate receptor (NMDAR) could have a pathogenic role during fetal brain development. Here we describe a maternal SLE mouse model wherein pregnant dams harbored DNA-specific, NMDAR-specific autoantibodies throughout gestation. High titers of these autoantibodies in maternal circulation led to histological abnormalities in fetal brain and subsequent cognitive impairments in adult offspring. These data support a paradigm in which in utero exposure to neurotoxic autoantibodies causes abnormal brain development with long-term consequences. This paradigm may apply to multiple congenital neuropsychiatric disorders.
Figures
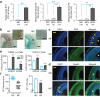

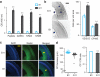
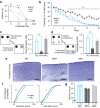
Similar articles
-
Neonatal lupus erythematosus in offspring of mothers with experimental systemic lupus erythematosus.Am J Reprod Immunol. 1992 Oct-Dec;28(3-4):264-8. doi: 10.1111/j.1600-0897.1992.tb00811.x. Am J Reprod Immunol. 1992. PMID: 1285898
-
Female mouse fetal loss mediated by maternal autoantibody.J Exp Med. 2012 Jun 4;209(6):1083-9. doi: 10.1084/jem.20111986. Epub 2012 May 7. J Exp Med. 2012. PMID: 22565825 Free PMC article.
-
Anti-prolactin autoantibodies in pregnant women with systemic lupus erythematosus: maternal and fetal outcome.Lupus. 2007;16(5):342-9. doi: 10.1177/0961203307078197. Lupus. 2007. PMID: 17576736
-
Neurodevelopmental disorders in children born to mothers with systemic lupus erythematosus.Lupus. 2014 Oct;23(11):1099-104. doi: 10.1177/0961203314541691. Epub 2014 Jun 26. Lupus. 2014. PMID: 24969080 Review.
-
Autoantibodies involved in neuropsychiatric manifestations associated with systemic lupus erythematosus.J Neuroimmunol. 2009 Jul 25;212(1-2):3-9. doi: 10.1016/j.jneuroim.2009.05.003. Epub 2009 Jun 4. J Neuroimmunol. 2009. PMID: 19500858 Review.
Cited by
-
Immune mediators in the brain and peripheral tissues in autism spectrum disorder.Nat Rev Neurosci. 2015 Aug;16(8):469-86. doi: 10.1038/nrn3978. Nat Rev Neurosci. 2015. PMID: 26189694 Free PMC article. Review.
-
Surface dynamics of GluN2B-NMDA receptors controls plasticity of maturing glutamate synapses.EMBO J. 2014 Apr 16;33(8):842-61. doi: 10.1002/embj.201386356. Epub 2014 Mar 3. EMBO J. 2014. PMID: 24591565 Free PMC article.
-
Antibodies in neurological diseases: Established, emerging, explorative.Immunol Rev. 2024 Nov;328(1):283-299. doi: 10.1111/imr.13405. Epub 2024 Oct 1. Immunol Rev. 2024. PMID: 39351782 Free PMC article. Review.
-
Polyreactive autoantibodies in systemic lupus erythematosus have pathogenic potential.J Autoimmun. 2009 Nov-Dec;33(3-4):270-4. doi: 10.1016/j.jaut.2009.03.011. Epub 2009 Apr 26. J Autoimmun. 2009. PMID: 19398190 Free PMC article.
-
Glutamate receptor biology and its clinical significance in neuropsychiatric systemic lupus erythematosus.Rheum Dis Clin North Am. 2010 Feb;36(1):187-201, x-xi. doi: 10.1016/j.rdc.2009.12.007. Rheum Dis Clin North Am. 2010. PMID: 20202599 Free PMC article.
References
-
- Lahita RG. Systemic lupus erythematosus: learning disability in the male offspring of female patients and relationship to laterality. Psychoneuroendocrinology. 1988;13:385–396. - PubMed
-
- McAllister DL, et al. The influence of systemic lupus erythematosus on fetal development: cognitive, behavioral, and health trends. J Int Neuropsychol Soc. 1997;3:370–376. - PubMed
-
- Neri F, et al. Neuropsychological development of children born to patients with systemic lupus erythematosus. Lupus. 2004;13:805–811. - PubMed
-
- Ross G, Sammaritano L, Nass R, Lockshin M. Effects of mothers' autoimmune disease during pregnancy on learning disabilities and hand preference in their children. Arch Pediatr Adolesc Med. 2003;157:397–402. - PubMed
-
- Tincani A, et al. Impact of in utero environment on the offspring of lupus patients. Lupus. 2006;15:801–807. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

