Hydrogen peroxide mobilizes Ca2+ through two distinct mechanisms in rat hepatocytes
- PMID: 19079290
- PMCID: PMC4006535
- DOI: 10.1038/aps.2008.4
Hydrogen peroxide mobilizes Ca2+ through two distinct mechanisms in rat hepatocytes
Abstract
Aim: Hydrogen peroxide (H2O2) is produced during liver transplantation. Ischemia/reperfusion induces oxidation and causes intracellular Ca2+ overload, which harms liver cells. Our goal was to determine the precise mechanisms of these processes.
Methods: Hepatocytes were extracted from rats. Intracellular Ca2+ concentrations ([Ca2+](i)), inner mitochondrial membrane potentials and NAD(P)H levels were measured using fluorescence imaging. Phospholipase C (PLC) activity was detected using exogenous PIP2. ATP concentrations were measured using the luciferin-luciferase method. Patch-clamp recordings were performed to evaluate membrane currents.
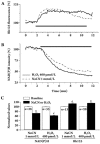
Results: H2O2 increased intracellular Ca2+ concentrations ([Ca2+](i)) across two kinetic phases. A low concentration (400 micromol/L) of H2O2 induced a sustained elevation of [Ca2+](i) that was reversed by removing extracellular Ca2+. H2O2 increased membrane currents consistent with intracellular ATP concentrations. The non-selective ATP-sensitive cation channel blocker amiloride inhibited H2O2-induced membrane current increases and [Ca2+](i) elevation. A high concentration (1 mmol/L)of H2O2 induced an additional transient elevation of [Ca2+](i), which was abolished by the specific PLC blocker U73122 but was not eliminated by removal of extracellular Ca2+. PLC activity was increased by 1 mmol/L H2O2 but not by 400 micromol/L H2O2.
Conclusions: H2O2 mobilizes Ca2+ through two distinct mechanisms. In one, 400 micromol/L H2O2-induced sustained [Ca2+](i) elevation is mediated via a Ca2+ influx mechanism, under which H2O2 impairs mitochondrial function via oxidative stress,reduces intracellular ATP production, and in turn opens ATP-sensitive, non-specific cation channels, leading to Ca2+ influx.In contrast, 1 mmol/L H2O2-induced transient elevation of [Ca2+](i) is mediated via activation of the PLC signaling pathway and subsequently, by mobilization of Ca2+ from intracellular Ca2+ stores.
Figures







References
-
- Bzeizi KI, Dawkes R, Dodd NJ, Plevris JN, Hayes PC. Graft dysfunction following liver transplantation: role of free radicals. J Hepatol. 1997;26:69–74. - PubMed
-
- Jaeschke H. Mechanisms of liver injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1083–8. - PubMed
-
- Rosser BG, Gores GJ. Liver cell necrosis: cellular mechanisms and clinical implications. Gastroenterology. 1995;108:252–75. - PubMed
-
- Rychkov G, Brereton HM, Harland ML, Barritt GJ. Plasma membrane Ca2+ release-activated Ca2+ channels with a high selectivity for Ca2+ identified by patch-clamp recording in rat liver cells. Hepatology. 2001;33:938–47. - PubMed
-
- Hoffman JW, Jr, Gilbert TB, Poston RS, Silldorff EP. Myocardial reperfusion injury: etiology, mechanisms, and therapies. J Extra Corpor Technol. 2004;36:391–411. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

