Transformation and action of extracellular NAD+ in perfused rat and mouse livers
- PMID: 19079292
- PMCID: PMC4006529
- DOI: 10.1038/aps.2008.7
Transformation and action of extracellular NAD+ in perfused rat and mouse livers
Abstract
Aim: Transformation and possible metabolic effects of extracellular NAD+ were investigated in the livers of mice (Mus musculus; Swiss strain) and rats (Rattus novergicus; Holtzman and Wistar strains).
Methods: The livers were perfused in an open system using oxygen-saturated Krebs/Henseleit-bicarbonate buffer (pH 7.4) as the perfusion fluid. The transformation of NAD+ was monitored using high-performance liquid chromatography.
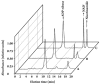
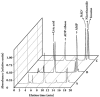
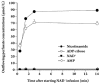
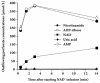
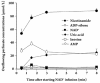
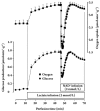
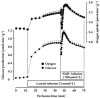
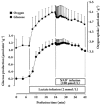
Results: In the mouse liver, the single-pass metabolism of 100 micromol/L NAD+ was almost complete; ADP-ribose and nicotinamide were the main products in the outflowing perfusate. In the livers of both Holtzman and Wistar rats, the main transformation products were ADP-ribose, uric acid and nicotinamide; significant amounts of inosine and AMP were also identified. On a weight basis, the transformation of NAD+ was more efficient in the mouse liver. In the rat liver, 100 micromol/L NAD+ transiently inhibited gluconeogenesis and oxygen uptake. Inhibition was followed by a transient stimulation. Inhibition was more pronounced in the Wistar strain and stimulation was more pronounced in the Holtzman strain. In the mouse liver, no clear effects on gluconeogenesis and oxygen uptake were found even at 500 micromol/L NAD+.
Conclusion: It can be concluded that the functions of extracellular NAD+ are species-dependent and that observations in one species are strictly valid for that species. Interspecies extrapolations should thus be made very carefully. Actually, even variants of the same species can demonstrate considerably different responses.
Figures
References
-
- Liersch M, Grotelüschen H, Decker K. NAD permeation into the liver cell. Hoppe-Seyler's Z Physiol Chem. 1971;352:267–74. - PubMed
-
- Ziegler M. New functions of a long-known molecule. Emerging roles of NAD in cellular signaling. Eur J Biochem. 2000;267:1550–64. - PubMed
-
- Rusinko N, Lee HC. Widespread occurrence in animal tissues of an enzyme catalyzing the conversion of NAD+ into a cyclic metabolite with intracellular Ca2+-mobilizing activity. J Biol Chem. 1989;264:11725–31. - PubMed
-
- Chini EN, Klener P, Jr, Beers KW, Chini CCS, Grande JP, Dousa TP. Cyclic ADP-ribose metabolism in rat kidney: high capacity for synthesis in glomeruli. Kidney Int. 1997;51:1500–6. - PubMed
-
- Khoo KM, Chang CF. Localization of plasma membrane CD38 is domain specific in rat hepatocyte. Arch Biochem Biophys. 2000;373:35–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

