Keratinocyte-derived follistatin regulates epidermal homeostasis and wound repair
- PMID: 19079322
- PMCID: PMC4087116
- DOI: 10.1038/labinvest.2008.120
Keratinocyte-derived follistatin regulates epidermal homeostasis and wound repair
Abstract
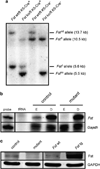
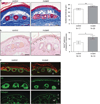
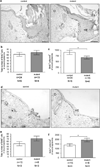
Activin is a growth and differentiation factor that controls development and repair of several tissues and organs. Transgenic mice overexpressing activin in the skin were characterized by strongly enhanced wound healing, but also by excessive scarring. In this study, we explored the consequences of targeted activation of activin in the epidermis and hair follicles by generation of mice lacking the activin antagonist follistatin in keratinocytes. We observed enhanced keratinocyte proliferation in the tail epidermis of these animals. After skin injury, an earlier onset of keratinocyte hyperproliferation at the wound edge was observed in the mutant mice, resulting in an enlarged hyperproliferative epithelium. However, granulation tissue formation and scarring were not affected. These results demonstrate that selective activation of activin in the epidermis enhances reepithelialization without affecting the quality of the healed wound.
Figures




References
-
- Phillips DJ. The activin/inhibin family. In: Thomson AW, Lotze MT, editors. The Cytokine Handbook. 4th edn. London: Academic Press; 2003. pp. 1153–1177.
-
- Mathews LS. Activin receptors and cellular signaling by the receptor serine kinase family. Endocr Rev. 1994;15:310–325. - PubMed
-
- Nakamura T, Takio K, Eto Y, et al. Activin-binding protein from rat ovary is follistatin. Science. 1990;247:836–838. - PubMed
-
- Chang H, Brown CW, Matzuk MM. Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr Rev. 2002;23:787–823. - PubMed
-
- Matzuk MM, Lu N, Vogel H, et al. Multiple defects and perinatal death in mice deficient in follistatin. Nature. 1995;374:360–363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

