Connectivity of the primate superior colliculus mapped by concurrent microstimulation and event-related FMRI
- PMID: 19079541
- PMCID: PMC2592545
- DOI: 10.1371/journal.pone.0003928
Connectivity of the primate superior colliculus mapped by concurrent microstimulation and event-related FMRI
Abstract
Background: Neuroanatomical studies investigating the connectivity of brain areas have heretofore employed procedures in which chemical or viral tracers are injected into an area of interest, and connected areas are subsequently identified using histological techniques. Such experiments require the sacrifice of the animals and do not allow for subsequent electrophysiological studies in the same subjects, rendering a direct investigation of the functional properties of anatomically identified areas impossible.
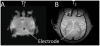
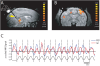
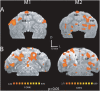
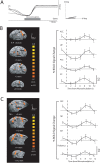
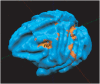
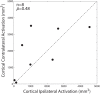
Methodology/principal findings: Here, we used a combination of microstimulation and fMRI in an anesthetized monkey preparation to study the connectivity of the superior colliculus (SC). Microstimulation of the SC resulted in changes in the blood oxygenation level-dependent (BOLD) signals in the SC and in several cortical and subcortical areas consistent with the known connectivity of the SC in primates.
Conclusions/significance: These findings demonstrates that the concurrent use of microstimulation and fMRI can be used to identify brain networks for further electrophysiological or fMRI investigation.
Conflict of interest statement
Figures


References
-
- Ungerleider LG, Desimone R. Cortical connections of visual area MT in the macaque. JComp Neurol. 1986;248:190. - PubMed
-
- Leichnetz GR, Spencer RF, Hardy SG, Astruc J. The prefrontal corticotectal projection in the monkey; an anterograde and retrograde horseradish peroxidase study. Neuroscience. 1981;6:1023. - PubMed
-
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. CerebCortex. 1991;1:1. - PubMed
-
- Boldogkoi Z, Sik A, Denes A, Reichart A, Toldi J, et al. Novel tracing paradigms–genetically engineered herpesviruses as tools for mapping functional circuits within the CNS: present status and future prospects. Prog Neurobiol. 2004;72:417–445. - PubMed
-
- Kobbert C, Apps R, Bechmann I, Lanciego JL, Mey J, et al. Current concepts in neuroanatomical tracing. Prog Neurobiol. 2000;62:327–351. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

