The nucleation and growth of calcium phosphate by amelogenin
- PMID: 19079557
- PMCID: PMC2597808
- DOI: 10.1016/j.jcrysgro.2007.02.035
The nucleation and growth of calcium phosphate by amelogenin
Abstract
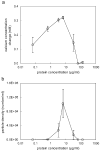
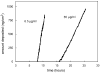
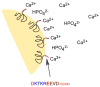
The nucleation processes involved in calcium phosphate formation in tooth enamel are not well understood but are believed to involve proteins in the extracellular matrix. The ability of one enamel protein, amelogenin, to promote the nucleation and growth of calcium phosphate was studied in an in vitro system involving metastable supersaturated solutions. It was found that recombinant amelogenin (rM179 and rp(H)M180) promoted the nucleation of calcium phosphate compared to solutions without protein. The amount of calcium phosphate increased with increasing supersaturation of the solutions and increasing protein concentrations up to 6.5 μg/mL. At higher protein concentrations, the amount of calcium phosphate decreased. The kinetics of nucleation was studied in situ and in real time using a quartz crystal microbalance (QCM) and showed that the protein reduced the induction time for nucleation compared to solutions without protein. This work shows a nucleation role for amelogenin in vitro which may be promoted by the association of amelogenin into nanosphere templates, exposing charged functionality at the surface.
Figures


References
-
- Lowenstam HA, Weiner S. On Biomineralization. Oxford University Press; New York: 1989.
-
- Fincham AG, Moradian-Oldak J, Simmer JP. J Struct Biol. 1999;136:270. - PubMed
-
- Moradian-Oldak J. J Matrix Biol. 2001;20:293. - PubMed
-
- Diekwisch TGH, Berman BJ, Gentner S, Slavkin HC. Cell Tissue Res. 1995;279:149. - PubMed
-
- Moradian-Oldak J, Tan J, Fincham AG. Biopolymers. 1998;46:225. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
