Encoding of naturalistic stimuli by local field potential spectra in networks of excitatory and inhibitory neurons
- PMID: 19079571
- PMCID: PMC2585056
- DOI: 10.1371/journal.pcbi.1000239
Encoding of naturalistic stimuli by local field potential spectra in networks of excitatory and inhibitory neurons
Abstract
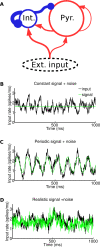
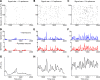
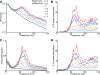
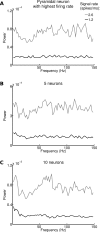
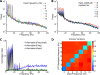
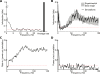
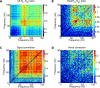
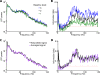
Recordings of local field potentials (LFPs) reveal that the sensory cortex displays rhythmic activity and fluctuations over a wide range of frequencies and amplitudes. Yet, the role of this kind of activity in encoding sensory information remains largely unknown. To understand the rules of translation between the structure of sensory stimuli and the fluctuations of cortical responses, we simulated a sparsely connected network of excitatory and inhibitory neurons modeling a local cortical population, and we determined how the LFPs generated by the network encode information about input stimuli. We first considered simple static and periodic stimuli and then naturalistic input stimuli based on electrophysiological recordings from the thalamus of anesthetized monkeys watching natural movie scenes. We found that the simulated network produced stimulus-related LFP changes that were in striking agreement with the LFPs obtained from the primary visual cortex. Moreover, our results demonstrate that the network encoded static input spike rates into gamma-range oscillations generated by inhibitory-excitatory neural interactions and encoded slow dynamic features of the input into slow LFP fluctuations mediated by stimulus-neural interactions. The model cortical network processed dynamic stimuli with naturalistic temporal structure by using low and high response frequencies as independent communication channels, again in agreement with recent reports from visual cortex responses to naturalistic movies. One potential function of this frequency decomposition into independent information channels operated by the cortical network may be that of enhancing the capacity of the cortical column to encode our complex sensory environment.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Cortical dynamics during naturalistic sensory stimulations: experiments and models.J Physiol Paris. 2011 Jan-Jun;105(1-3):2-15. doi: 10.1016/j.jphysparis.2011.07.014. Epub 2011 Aug 31. J Physiol Paris. 2011. PMID: 21907800
-
Stimulus dependence of local field potential spectra: experiment versus theory.J Neurosci. 2014 Oct 29;34(44):14589-605. doi: 10.1523/JNEUROSCI.5365-13.2014. J Neurosci. 2014. PMID: 25355213 Free PMC article.
-
Low-frequency local field potentials and spikes in primary visual cortex convey independent visual information.J Neurosci. 2008 May 28;28(22):5696-709. doi: 10.1523/JNEUROSCI.0009-08.2008. J Neurosci. 2008. PMID: 18509031 Free PMC article.
-
Pattern separation and synchronization in spiking associative memories and visual areas.Neural Netw. 2001 Jul-Sep;14(6-7):763-80. doi: 10.1016/s0893-6080(01)00084-3. Neural Netw. 2001. PMID: 11665769 Review.
-
Local networks in visual cortex and their influence on neuronal responses and dynamics.J Physiol Paris. 2004 Jul-Nov;98(4-6):429-41. doi: 10.1016/j.jphysparis.2005.09.017. Epub 2005 Nov 7. J Physiol Paris. 2004. PMID: 16274974 Review.
Cited by
-
Computation of the electroencephalogram (EEG) from network models of point neurons.PLoS Comput Biol. 2021 Apr 2;17(4):e1008893. doi: 10.1371/journal.pcbi.1008893. eCollection 2021 Apr. PLoS Comput Biol. 2021. PMID: 33798190 Free PMC article.
-
A model of the differential representation of signal novelty in the local field potentials and spiking activity of the ventrolateral prefrontal cortex.Neural Comput. 2013 Jan;25(1):157-85. doi: 10.1162/NECO_a_00388. Epub 2012 Sep 28. Neural Comput. 2013. PMID: 23020111 Free PMC article.
-
Criticality enhances the multilevel reliability of stimulus responses in cortical neural networks.PLoS Comput Biol. 2022 Jan 31;18(1):e1009848. doi: 10.1371/journal.pcbi.1009848. eCollection 2022 Jan. PLoS Comput Biol. 2022. PMID: 35100254 Free PMC article.
-
Hebbian reverberations in emotional memory micro circuits.Front Neurosci. 2009 Sep 15;3(2):198-205. doi: 10.3389/neuro.01.027.2009. eCollection 2009 Sep. Front Neurosci. 2009. PMID: 20011142 Free PMC article.
-
Intracellular Dynamics in Cuneate Nucleus Neurons Support Self-Stabilizing Learning of Generalizable Tactile Representations.Front Cell Neurosci. 2018 Jul 31;12:210. doi: 10.3389/fncel.2018.00210. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30108485 Free PMC article.
References
-
- Neville KR, Haberly LB. Beta and gamma oscillations in the olfactory system of the urethane-anesthetized rat. J Neurophysiol. 2003;90:3921–3930. - PubMed
-
- Brosch M, Budinger E, Scheich H. Stimulus-related gamma oscillations in primate auditory cortex. J Neurophysiol. 2002;87:2715–2725. - PubMed
-
- Kayser C, Petkov CI, Logothetis NK. Tuning to sound frequency in auditory field potentials. J Neurophysiol. 2007;98:1806–1809. - PubMed
-
- Gray CM, Konig P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–337. - PubMed
-
- Rols G, Tallon-Baudry C, Girard P, Bertrand O, Bullier J. Cortical mapping of gamma oscillations in areas V1 and V4 of the macaque monkey. Vis Neurosci. 2001;18:527–540. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

