Immune mechanisms responsible for vaccination against and clearance of mucosal and lymphatic norovirus infection
- PMID: 19079577
- PMCID: PMC2587711
- DOI: 10.1371/journal.ppat.1000236
Immune mechanisms responsible for vaccination against and clearance of mucosal and lymphatic norovirus infection
Abstract
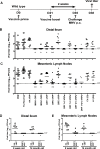
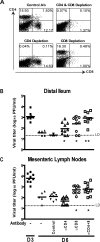
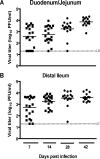
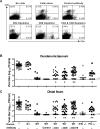
Two cardinal manifestations of viral immunity are efficient clearance of acute infection and the capacity to vaccinate against secondary viral exposure. For noroviruses, the contributions of T cells to viral clearance and vaccination have not been elucidated. We report here that both CD4 and CD8 T cells are required for efficient clearance of primary murine norovirus (MNV) infection from the intestine and intestinal lymph nodes. Further, long-lasting protective immunity was generated by oral live virus vaccination. Systemic vaccination with the MNV capsid protein also effectively protected against mucosal challenge, while vaccination with the capsid protein of the distantly related human Lordsdale virus provided partial protection. Fully effective vaccination required a broad immune response including CD4 T cells, CD8 T cells, and B cells, but the importance of specific immune cell types varied between the intestine and intestinal lymph nodes. Perforin, but not interferon gamma, was required for clearance of MNV infection by adoptively transferred T lymphocytes from vaccinated hosts. These studies prove the feasibility of both mucosal and systemic vaccination against mucosal norovirus infection, demonstrate tissue specificity of norovirus immune cells, and indicate that efficient vaccination strategies should induce potent CD4 and CD8 T cell responses.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Estes MK, Prasad BV, Atmar RL. Noroviruses everywhere: Has something changed? Curr Opin Infect Dis. 2006;19:467–474. - PubMed
-
- Fankhauser RL, Noel JS, Monroe SS, Ando T, Glass RI. Molecular epidemiology of “Norwalk-like viruses” in outbreaks of gastroenteritis in the United States. J Infect Dis. 1998;178:1571–1578. - PubMed
-
- Fankhauser RL, Monroe SS, Noel JS, Humphrey CD, Bresee JS, et al. Epidemiologic and molecular trends of “Norwalk-like viruses” associated with outbreaks of gastroenteritis in the United States. J Infect Dis. 2002;186:1–7. - PubMed
-
- Green KY, Chanock RM, Kapikian AZ. Human Caliciviruses. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 841–874.
-
- Centers for Disease Control and Prevention (CDC) Norovirus activity—United States, 2006–2007. MMWR Morb Mortal Wkly Rep. 2007;56:842–846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

