Phosphorylation by Cdk1 increases the binding of Eg5 to microtubules in vitro and in Xenopus egg extract spindles
- PMID: 19079595
- PMCID: PMC2592692
- DOI: 10.1371/journal.pone.0003936
Phosphorylation by Cdk1 increases the binding of Eg5 to microtubules in vitro and in Xenopus egg extract spindles
Abstract
Background: Motor proteins from the kinesin-5 subfamily play an essential role in spindle assembly during cell division of most organisms. These motors crosslink and slide microtubules in the spindle. Kinesin-5 motors are phosphorylated at a conserved site by Cyclin-dependent kinase 1 (Cdk1) during mitosis. Xenopus laevis kinesin-5 has also been reported to be phosphorylated by Aurora A in vitro.
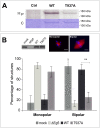
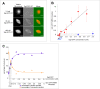
Methodology/principal findings: We investigate here the effect of these phosphorylations on kinesin-5 from Xenopus laevis, called Eg5. We find that phosphorylation at threonine 937 in the C-terminal tail of Eg5 by Cdk1 does not affect the velocity of Eg5, but strongly increases its binding to microtubules assembled in buffer. Likewise, this phosphorylation promotes binding of Eg5 to microtubules in Xenopus egg extract spindles. This enhancement of binding elevates the amount of Eg5 in spindles above a critical level required for bipolar spindle formation. We find furthermore that phosphorylation of Xenopus laevis Eg5 by Aurora A at serine 543 in the stalk is not required for spindle formation.
Conclusions/significance: These results show that phosphorylation of Eg5 by Cdk1 has a direct effect on the interaction of this motor with microtubules. In egg extract, phosphorylation of Eg5 by Cdk1 ensures that the amount of Eg5 in the spindle is above a level that is required for spindle formation. This enhanced targeting to the spindle appears therefore to be, at least in part, a direct consequence of the enhanced binding of Eg5 to microtubules upon phosphorylation by Cdk1. These findings advance our understanding of the regulation of this essential mitotic motor protein.
Conflict of interest statement
Figures


References
-
- Murray AW. Recycling the cell cycle: cyclins revisited. Cell. 2004;116:221–234. - PubMed
-
- Malumbres M, Barbacid M. Cell cycle kinases in cancer. Curr Opin Genet Dev. 2007;17:60–65. - PubMed
-
- Hildebrandt ER, Hoyt MA. Mitotic motors in Saccharomyces cerevisiae. Biochim Biophys Acta. 2000;1496:99–116. - PubMed
-
- Sharp DJ, Rogers GC, Scholey JM. Microtubule motors in mitosis. Nature. 2000;407:41–47. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

