Morphoproteomic evidence of constitutively activated and overexpressed mTOR pathway in cervical squamous carcinoma and high grade squamous intraepithelial lesions
- PMID: 19079619
- PMCID: PMC2600462
Morphoproteomic evidence of constitutively activated and overexpressed mTOR pathway in cervical squamous carcinoma and high grade squamous intraepithelial lesions
Abstract
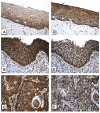
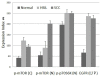
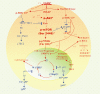
Human papilloma virus (HPV) infection of the uterine cervix is linked to the pathogenesis of cervical cancer. Preclinical in vitro and in vivo studies using HPV-containing human cervical carcinoma cell lines have shown that the mammalian target of rapamycin (mTOR) inhibitor, rapamycin, and epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor, erlotinib, can induce growth delay of xenografts. Activation of Akt and mTOR are also observed in cervical squamous cell carcinoma and, the expression of phosphorylated mTOR was reported to serve as a marker to predict response to chemotherapy and survival of cervical cancer patients. Therefore, we investigated: a) the expression level of EGFR in cervical squamous cell carcinoma (SCC) and high-grade squamous intraepithelial lesions (HSIL) versus non-neoplastic cervical squamous epithelium; b) the state of activation of the mTOR pathway in these same tissues; and c) any impact of these signal transduction molecules on cell cycle. Formalin-fixed paraffin-embedded tissue microarray blocks containing 20 samples each of normal cervix, HSIL and invasive SCC, derived from a total of 60 cases of cervical biopsies and cervical conizations were examined. Immunohistochemistry was utilized to detect the following antigens: EGFR; mTOR pathway markers, phosphorylated (p)-mTOR (Ser2448) and p-p70S6K (Thr389); and cell cycle associated proteins, Ki-67 and S phase kinase-associated protein (Skp)2. Protein compartmentalization and expression were quantified in regard to proportion (0-100%) and intensity (0-3+). Mitotic index (MI) was also assessed. An expression index (EI) for pmTOR, p-p70S6K and EGFR, respectively was calculated by taking the product of intensity score and proportion of positively staining cells. We found that plasmalemmal EGFR expression was limited to the basal/parabasal cells (2-3+, EI = 67) in normal cervical epithelium (NL), but was diffusely positive in all HSIL (EI = 237) and SCC (EI 226). The pattern of cytoplasmic p-mTOR and nuclear p-p70S6K expression was similar to that of EGFR; all showed a significantly increased EI in HSIL/SCC versus NL (p<0.02). Nuclear translocation of p-mTOR was observed in all SCC lesions (EI = 202) and was significantly increased versus both HSIL (EI = 89) and NL (EI = 54) with p<0.015 and p<0.0001, respectively. Concomitant increases in MI and proportion of nuclear Ki-67 and Skp2 expression were noted in HSIL and SCC. In conclusion, morphoproteomic analysis reveals constitutive activation and overexpression of the mTOR pathway in HSIL and SCC as evidenced by: increased nuclear translocation of pmTOR and p-p70S6K, phosphorylated at putative sites of activation, Ser2448 and Thr389, respectively; correlative overexpression of the upstream signal transducer, EGFR, and increases in cell cycle correlates, Skp2 and mitotic indices. These results suggest that the mTOR pathway plays a key role in cervical carcinogenesis and targeted therapies may be developed for SCC as well as its precursor lesion, HSIL.
Keywords: cervical squamous carcinoma; high grade SIL; mTOR pathway; morphoproteomics.
Figures


Similar articles
-
Morphoproteomic profile of mTOR, Ras/Raf kinase/ERK, and NF-kappaB pathways in human gastric adenocarcinoma.Ann Clin Lab Sci. 2008 Summer;38(3):195-209. Ann Clin Lab Sci. 2008. PMID: 18715846
-
Morphoproteomic and pharmacoproteomic rationale for mTOR effectors as therapeutic targets in head and neck squamous cell carcinoma.Ann Clin Lab Sci. 2006 Summer;36(3):273-82. Ann Clin Lab Sci. 2006. PMID: 16951268
-
Morphoproteomic and molecular concomitants of an overexpressed and activated mTOR pathway in renal cell carcinomas.Ann Clin Lab Sci. 2006 Summer;36(3):283-93. Ann Clin Lab Sci. 2006. PMID: 16951269
-
Squamous intraepithelial lesions (SIL: LSIL, HSIL, ASCUS, ASC-H, LSIL-H) of Uterine Cervix and Bethesda System.Cytojournal. 2021 Jul 17;18:16. doi: 10.25259/Cytojournal_24_2021. eCollection 2021. Cytojournal. 2021. PMID: 34345247 Free PMC article. Review.
-
Anal Cancer in High-Risk Women: The Lost Tribe.Cancers (Basel). 2022 Dec 22;15(1):60. doi: 10.3390/cancers15010060. Cancers (Basel). 2022. PMID: 36612055 Free PMC article. Review.
Cited by
-
Expression and clinical significance of mammalian target of rapamycin/P70 ribosomal protein S6 kinase signaling pathway in human colorectal carcinoma tissue.Oncol Lett. 2015 Jul;10(1):277-282. doi: 10.3892/ol.2015.3228. Epub 2015 May 19. Oncol Lett. 2015. PMID: 26171014 Free PMC article.
-
Morphoproteomics, E6/E7 in-situ hybridization, and biomedical analytics define the etiopathogenesis of HPV-associated oropharyngeal carcinoma and provide targeted therapeutic options.J Otolaryngol Head Neck Surg. 2017 Aug 17;46(1):52. doi: 10.1186/s40463-017-0230-2. J Otolaryngol Head Neck Surg. 2017. PMID: 28818106 Free PMC article.
-
Activation of cap-dependent translation by mucosal human papillomavirus E6 proteins is dependent on the integrity of the LXXLL binding motif.J Virol. 2012 Jul;86(14):7466-72. doi: 10.1128/JVI.00487-12. Epub 2012 May 2. J Virol. 2012. PMID: 22553330 Free PMC article.
-
Reduced expression of autophagy markers correlates with high-risk human papillomavirus infection in human cervical squamous cell carcinoma.Oncol Lett. 2014 Oct;8(4):1492-1498. doi: 10.3892/ol.2014.2417. Epub 2014 Aug 5. Oncol Lett. 2014. PMID: 25202355 Free PMC article.
-
Prevention of tumor growth driven by PIK3CA and HPV oncogenes by targeting mTOR signaling with metformin in oral squamous carcinomas expressing OCT3.Cancer Prev Res (Phila). 2015 Mar;8(3):197-207. doi: 10.1158/1940-6207.CAPR-14-0348. Epub 2015 Feb 13. Cancer Prev Res (Phila). 2015. PMID: 25681087 Free PMC article.
References
-
- Rosai J, editor. ROSAI AND ACKERMAN’S Surgical Pathology. 9th edition. Philadelphia, USA (Printed in China): Mosby, An Affiliate of Elsevier Inc; 2004. pp. 1532–1539.
-
- Jiang M, Milner J. Selective silencing of viral gene expression in HPV-positive human cervical carcinoma cells treated with siRNA, a primer of RNA interference. Oncogene. 2002;21:6041–6048. - PubMed
-
- Jiang M, Milner J. Selective silencing of viral gene E6 and E7 expression in HPV-positive human cervical carcinoma cells using small interfering RNAs. Methods Mol Biol. 2005;292:401–420. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
