Alteration of Drug Sensitivity in Human Colon Cancer Cells after Exposure to Heat: Implications for Liver Metastasis Therapy using RFA and Chemotherapy
- PMID: 19079666
- PMCID: PMC2596320
Alteration of Drug Sensitivity in Human Colon Cancer Cells after Exposure to Heat: Implications for Liver Metastasis Therapy using RFA and Chemotherapy
Abstract
Radiofrequency ablation (RFA) is gaining popularity for treating colorectal liver metastases by inducing image guided tumor hyperthermia. In order to reduce tumor recurrence, adjuvant therapies have been administered post-RFA. We hypothesized that tumor cells escaping RFA cytotoxicity by being in the sublethal zones of tumor might develop differential behavior toward cytotoxic drugs. Here, we used cultured human colorectal cancer cells to evaluate the interaction between heat treatment and chemotherapeutic agents. Human colon cancer cell lines HT29 and HCT116 were subjected to temperatures of 42 degrees to 50 degrees C for 15 min, in combination with 5-fluorouracil, oxaliplatin, or irinotecan at different sequences. Cytotoxicity was determined by MTT assay. The cell cycle progression was analyzed by flow cytometry with propidium iodide staining. The expression of several genes associated with drug sensitivity was quantitated by real-time RT-PCR before and after heat treatment. Either heat treatment at 45 degrees C by simultaneous or pre-treatment with three different chemotherapeutic agents didn't affect the cytotoxicity of the combined treatment to HT29 and HCT116 cells, except for irinotecan treatment in HCT116 cells. However, when pre-exposure to 45 degrees C, HCT116 cells, but not HT29 cells, developed resistance to these three drugs. In an analysis of cell cycle profile after the drug followed heat treatment, a longer delay in cell cycle progression in HCT116 cells was observed in comparison to HT29 cells. Furthermore, HCT116 and HT29 cells exhibited different expression profiles of several drug-related genes in response to heat treatment at 45 degrees C. An observation of a differential response to the drug and heat treatment sequences between two human colon cancer cell lines suggests that tumor heterogeneity and selection of chemotherapeutic agents need to be under consideration in the clinical setting.
Keywords: Hyperthermia; cell cycle; chemotherapy; colon cancer; drug resistance; gene expression.
Figures
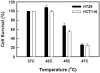
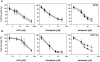
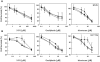

 ) and 45°C (▪) for 15 min. Drugs were removed form medium before heat treatment. Cell viability was determined by MTT assay 72 h after heat treatment. Data are shown as mean ± SD. *p < 0.05, compared to cells at 37°C by the Student's test. Cell survival for each termperature was corrected for heat cytotoxicity.
) and 45°C (▪) for 15 min. Drugs were removed form medium before heat treatment. Cell viability was determined by MTT assay 72 h after heat treatment. Data are shown as mean ± SD. *p < 0.05, compared to cells at 37°C by the Student's test. Cell survival for each termperature was corrected for heat cytotoxicity.

References
-
- Stangl R, Altendorf-Hofmann Hofmann, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343:1405–1410. - PubMed
-
- Fong Y, Cohen AM, Fortner JG, Enker WE, Turnbull AD, Coit DG, Marrero AM, Prasad M, Blumgart LH, Brennan MF. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–946. - PubMed
-
- Hoff PM, Ansari R, Batist G, Cox J, Kocha W, Kuperminc M, Maroun J, Walde D, Weaver C, Harrison E, Burger HU, Osterwalder B, Wong AO, Wong R. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001;19:2282–2292. - PubMed
-
- Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–914. - PubMed
LinkOut - more resources
Full Text Sources
