Copper Induces Apoptosis of Neuroblastoma Cells Via Post-translational Regulation of the Expression of Bcl-2-family Proteins and the tx Mouse is a Better Model of Hepatic than Brain Cu Toxicity
- PMID: 19079689
- PMCID: PMC2596338
Copper Induces Apoptosis of Neuroblastoma Cells Via Post-translational Regulation of the Expression of Bcl-2-family Proteins and the tx Mouse is a Better Model of Hepatic than Brain Cu Toxicity
Abstract
The basic mechanism(s) by which altered Cu homeostasis is toxic to hepatocytes and neurons, the two major cell types affected in copper storage diseases such as Wilson's disease (WD), remain unclear. Using human M17 neuroblastoma cells as a model to examine Cu toxicity, we found that there was a time- and concentration-dependent induction of neuronal death, such that at 24 h there was a approximately 50 % reduction in viability with 25 muM Cu-glycine(2). Cu-glycine(2) (25:50 muM) treatment for 24 h significantly altered the expression of 296 genes, including 8 genes involved with apoptosis (BCL2-associated athanogene 3, BCL2/adenovirus E1B 19kDa interacting protein caspase 5, regulator of Fas-induced apoptosis, V-jun sarcoma virus 17 oncogene homolog, claudin 5, prostaglandin E receptor 3 and protein tyrosine phosphatase, non-receptor type 6). Surprisingly, changes in the expression of more 'traditional' apoptotic genes (Bcl-2, Bax, Bak and Bad) did not vary more than 20 %. To test whether the induction of apoptosis in neuroblastoma cells was via post-translational mechanisms, we measured the protein expression of these apoptotic markers in M17 neuroblastoma cells treated with Cu-glycine(2) (0-100 muM) for 24-48 h. Compared with glycine treated cells, Cu-glycine(2) reduced Bcl-2 expression by 50 %, but increased Bax and Bak expression by 130% and 400 %, respectively. To assess whether Cu also induced apoptotic cell death in a mouse model of WD, we measured the expression of these apoptotic markers in the liver and brain of mice expressing an ATP7b gene mutation (tx(J) mice) at 10 months of age (near the end of their lives when overt liver pathology is displayed). Changes in the liver expression of these apoptotic markers in tx(J) mice compared to background mice mirrored those of Cu treated neuroblastoma cells. In contrast, few changes in apoptotic protein expression were detected in the brain between tx(J) and background mice, indicating the tx(J) mouse is a good model of hepatic, but not brain, Cu toxicity. Our results indicate that Cu-induction of neuronal apoptosis does not require de novo synthesis or degradation of apoptotic genes, and that Cu accumulation in the aged tx(J) mouse brain is insufficient to induce apoptosis.
Keywords: Bad; Bak; Bax; Bcl-2; Copper; Wilson's disease; apoptosis; gene expression; glycine; metal ion; neuron; protein expression; toxic milk mice.
Figures

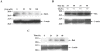

Similar articles
-
Copper Induces Oxidative Stress and Apoptosis in the Mouse Liver.Oxid Med Cell Longev. 2020 Jan 11;2020:1359164. doi: 10.1155/2020/1359164. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32411316 Free PMC article.
-
Copper deposition in Wilson's disease causes male fertility decline by impairing reproductive hormone release through inducing apoptosis and inhibiting ERK signal in hypothalamic-pituitary of mice.Front Endocrinol (Lausanne). 2022 Aug 5;13:961748. doi: 10.3389/fendo.2022.961748. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35992126 Free PMC article.
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
-
Wilson's disease: changes in methionine metabolism and inflammation affect global DNA methylation in early liver disease.Hepatology. 2013 Feb;57(2):555-65. doi: 10.1002/hep.26047. Epub 2013 Jan 10. Hepatology. 2013. PMID: 22945834 Free PMC article.
-
Metallothionein and apoptosis in the toxic milk mutant mouse.Lab Invest. 1998 Feb;78(2):175-83. Lab Invest. 1998. PMID: 9484715 Review.
Cited by
-
Polyphenol effects on CuO-nanoparticle-mediated DNA damage, reactive oxygen species generation, and fibroblast cell death.Toxicol In Vitro. 2022 Feb;78:105252. doi: 10.1016/j.tiv.2021.105252. Epub 2021 Oct 5. Toxicol In Vitro. 2022. PMID: 34624480 Free PMC article.
-
Copper Induces Oxidative Stress and Apoptosis in the Mouse Liver.Oxid Med Cell Longev. 2020 Jan 11;2020:1359164. doi: 10.1155/2020/1359164. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32411316 Free PMC article.
-
Copper Dependent Modulation of α-Synuclein Phosphorylation in Differentiated SHSY5Y Neuroblastoma Cells.Int J Mol Sci. 2021 Feb 18;22(4):2038. doi: 10.3390/ijms22042038. Int J Mol Sci. 2021. PMID: 33670800 Free PMC article.
-
Copper as a target for prostate cancer therapeutics: copper-ionophore pharmacology and altering systemic copper distribution.Oncotarget. 2016 Jun 14;7(24):37064-37080. doi: 10.18632/oncotarget.9245. Oncotarget. 2016. PMID: 27175597 Free PMC article.
-
Metal-Based Nanoparticles and Their Relevant Consequences on Cytotoxicity Cascade and Induced Oxidative Stress.Antioxidants (Basel). 2023 Mar 12;12(3):703. doi: 10.3390/antiox12030703. Antioxidants (Basel). 2023. PMID: 36978951 Free PMC article. Review.
References
-
- Andreoni G., Angeretti N., Lucca E., Forloni G. Densitometric quantification of neuronal viability by computerized image analysis. Exp Neurol. 1997;148:281–7. - PubMed
-
- Andrews N.C. Metal transporters and disease, Curr Opin Chem Biol. 2002;6:181–6. - PubMed
-
- Armendariz A.D., Gonzalez M., Loguinov A.V., Vulpe C.D. Gene expression profiling in chronic copper overload reveals upregulation and App. Physiol Genomics. 2004;20:45–54. - PubMed
-
- Atwood C.S., Huang X., Moir R.D., Tanzi R.E., Bush A.I. Role of free radicals and metal ions in the pathogenesis of Alzheimer's disease. Met Ions Biol Syst. 1999;36:309–64. - PubMed
-
- Atwood C.S., Scarpa R.C., Huang X., Moir R.D., Jones W.D., Fairlie D.P., Tanzi R.E., Bush A.I. Characterization of copper interactions with alzheimer amyloid beta peptides: identification of an attomolar-affinity copper binding site on amyloid beta1-42. J Neurochem. 2000;75:1219–33. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
