Population physiologically based pharmacokinetic modeling for the human lactational transfer of PCB-153 with consideration of worldwide human biomonitoring results
- PMID: 19079712
- PMCID: PMC2599755
- DOI: 10.1289/ehp.11519
Population physiologically based pharmacokinetic modeling for the human lactational transfer of PCB-153 with consideration of worldwide human biomonitoring results
Abstract
Background: One of the most serious human health concerns related to environmental contamination with polychlorinated biphenyls (PCBs) is the presence of these chemicals in breast milk.
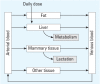
Objectives: We developed a physiologically based pharmacokinetic model of PCB-153 in women, and predict its transfer via lactation to infants. The model is the first human, population-scale lactational model for PCB-153. Data in the literature provided estimates for model development and for performance assessment.
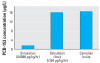
Methods: We used physiologic parameters from a cohort in Taiwan and reference values given in the literature to estimate partition coefficients based on chemical structure and the lipid content in various body tissues. Using exposure data from Japan, we predicted acquired body burden of PCB-153 at an average childbearing age of 25 years and compared predictions to measurements from studies in multiple countries. We attempted one example of reverse dosimetry modeling using our PBPK model for possible exposure scenarios in Canadian Inuits, the population with the highest breast milk PCB-153 level in the world.
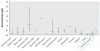
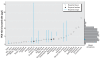
Results: Forward-model predictions agree well with human biomonitoring measurements, as represented by summary statistics and uncertainty estimates.
Conclusion: The model successfully describes the range of possible PCB-153 dispositions in maternal milk, suggesting a promising option for back-estimating doses for various populations.
Keywords: PCB-153; body burden; exposure reconstruction; human milk biomonitoring; lactational transfer; physiologically based pharmacokinetic modeling; polychlorinated biphenyls; reverse dosimetry.
Figures


References
-
- Abraham K, Papke O, Gross A, Kordonouri O, Wiegand S, Wahn U, et al. Time course of PCDD/PCDF/PCB concentrations in breast-feeding mothers and their infants. Chemosphere. 1998;37:1731–1741. - PubMed
-
- Akutsu K, Kuwabara K, Konishi Y, Matsumoto H, Murakami Y, Tanaka Y, et al. Congener-specific analysis of PCBs in food samples by using GC/MS [in Japanese] Shokuhin Eiseigaku Zasshi. 2005;46:99–108. - PubMed
-
- Allen BC, Hack CE, Clewell HJ. Use of Markov Chain Monte Carlo analysis with a physiologically-based pharmaco-kinetic model of methylmercury to estimate exposures in US women of childbearing age. Risk Anal. 2007;27:947–959. - PubMed
-
- Angulo R, Martinez P, Jodral ML. PCB congeners transferred by human milk, with an estimate of their daily intake. Food Chem Toxicol. 1999;37:1081–1088. - PubMed
-
- Brambilla G, Fochi I, Falce M, De Filippis SP, Ubaldi A, di Domenico A. PCDD and PCDF depletion in milk from dairy cows according to the herd metabolic scenario. Chemosphere. 2008;73(1 suppl):S216–S219. - PubMed
