Zirconium Bis(Amido) Catalysts for Asymmetric Intramolecular Alkene Hydroamination
- PMID: 19079735
- PMCID: PMC2600731
- DOI: 10.1021/om0606791
Zirconium Bis(Amido) Catalysts for Asymmetric Intramolecular Alkene Hydroamination
Abstract
In situ combination of diphosphinic amides and Zr(NMe(2))(4) results in the formation of chiral zirconium bis(amido) complexes. The complexes are competent catalysts for intramolecular asymmetric alkene hydroamintion, providing piperidines and pyrrolidines in up to 80% ee and high yield. This system utilizes an inexpensive zirconium precatalyst and readily prepared ligands and is the first asymmetric alkene hydroamination catalyst based upon a neutral zirconium bis(amido) complex.
Figures



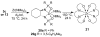
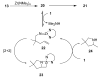
References
-
- Gagne MR, Brard L, Conticello VP, Giardello MA, Stern CL, Marks TJ. Organometallics. 1992;11:2003–2005.
-
-
For reviews of asymmetric hydroamination, see: (a) Togni, A.; Dorta, R.; Kollner, C.; Pioda, G. 1998, 70, 1477–1485.
- Togni A, Dorta R, Kollner C, Pioda G. 1998;70:1477–1485.
- Roesky PW, Muller TE. Angew Chem Int Ed. 2003;42:2708–2710. - PubMed
- Hong S, Marks TJ. Acc Chem Res. 2004;37:673–686. - PubMed
- Hultzsch KC. Org Biomol Chem. 2005;3:1819–1824. - PubMed
- Hultzsch KC. Adv Synth Catal. 2005;347:367–391.
- Hii KK. Pure Appl Chem. 2006;78:341–349.
-
-
-
For recent reports of hydroaminations using lanthanum and Group 3 metal complexes, see:
- Kim JY, Livinghouse T. Org Lett. 2005;7:1737–1739. - PubMed
- Collin J, Daran J, Jacquet O, Schulz E, Trifonov A. Chem Eur J. 2005;11:3455–3462. - PubMed
- Birkov DV, Hultzsch KC, Hampel F. J Am Chem Soc. 2006;128:3748–3759. - PubMed
- Riegert D, Collin J, Meddour A, Schulz E, Trifonov A. J Org Chem. 2006;71:2514–2517. - PubMed
-
-
-
For examples of asymmetric hydroaminations using other metals, including cationic zirconium complexes, see:
- Knight PD, Munslow I, O'Shaughnessy PN, Scott P. Chem Commun. 2004:894–895. - PubMed
- Gribkov DV, Hultzcsh KC. Angew Chem Int Ed. 2004;43:5542–5546. - PubMed
- Martinez PH, Hultzsch KC, Hampel F. Chem Commun. 2006:2221–2223. - PubMed
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
