Destabilization of the postsynaptic density by PSD-95 serine 73 phosphorylation inhibits spine growth and synaptic plasticity
- PMID: 19081375
- PMCID: PMC2671083
- DOI: 10.1016/j.neuron.2008.10.014
Destabilization of the postsynaptic density by PSD-95 serine 73 phosphorylation inhibits spine growth and synaptic plasticity
Erratum in
- Neuron. 2009 Jan 15;61(1):152
Abstract
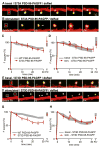
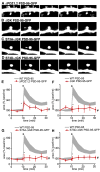
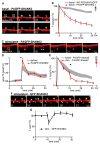
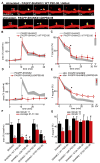
Long-term potentiation (LTP) is accompanied by dendritic spine growth and changes in the composition of the postsynaptic density (PSD). We find that activity-dependent growth of apical spines of CA1 pyramidal neurons is accompanied by destabilization of the PSD that results in transient loss and rapid replacement of PSD-95 and SHANK2. Signaling through PSD-95 is required for activity-dependent spine growth and trafficking of SHANK2. N-terminal PDZ and C-terminal guanylate kinase domains of PSD-95 are required for both processes, indicating that PSD-95 coordinates multiple signals to regulate morphological plasticity. Activity-dependent trafficking of PSD-95 is triggered by phosphorylation at serine 73, a conserved calcium/calmodulin-dependent protein kinase II (CaMKII) consensus phosphorylation site, which negatively regulates spine growth and potentiation of synaptic currents. We propose that PSD-95 and CaMKII act at multiple steps during plasticity induction to initially trigger and later terminate spine growth by trafficking growth-promoting PSD proteins out of the active spine.
Figures

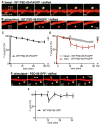
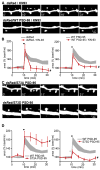
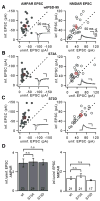
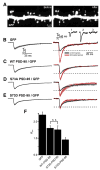
References
-
- Baron MK, Boeckers TM, Vaida B, Faham S, Gingery M, Sawaya MR, Salyer D, Gundelfinger ED, Bowie JU. An architectural framework that may lie at the core of the postsynaptic density. Science. 2006;311:531–535. - PubMed
-
- Bloodgood BL, Sabatini BL. Neuronal activity regulates diffusion across the neck of dendritic spines. Science. 2005;310:866–869. - PubMed
-
- Bockers TM, Mameza MG, Kreutz MR, Bockmann J, Weise C, Buck F, Richter D, Gundelfinger ED, Kreienkamp HJ. Synaptic scaffolding proteins in rat brain. Ankyrin repeats of the multidomain Shank protein family interact with the cytoskeletal protein alpha-fodrin. J Biol Chem. 2001;276:40104–40112. - PubMed
-
- Boeckers TM. The postsynaptic density. Cell Tissue Res. 2006;326:409–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

