Deregulation of HDAC1 by p25/Cdk5 in neurotoxicity
- PMID: 19081376
- PMCID: PMC2912147
- DOI: 10.1016/j.neuron.2008.10.015
Deregulation of HDAC1 by p25/Cdk5 in neurotoxicity
Abstract
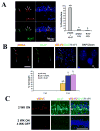
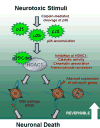
Aberrant cell-cycle activity and DNA damage are emerging as important pathological components in various neurodegenerative conditions. However, their underlying mechanisms are poorly understood. Here, we show that deregulation of histone deacetylase 1 (HDAC1) activity by p25/Cdk5 induces aberrant cell-cycle activity and double-strand DNA breaks leading to neurotoxicity. In a transgenic model for neurodegeneration, p25/Cdk5 activity elicited cell-cycle activity and double-strand DNA breaks that preceded neuronal death. Inhibition of HDAC1 activity by p25/Cdk5 was identified as an underlying mechanism for these events, and HDAC1 gain of function provided potent protection against DNA damage and neurotoxicity in cultured neurons and an in vivo model for ischemia. Our findings outline a pathological signaling pathway illustrating the importance of maintaining HDAC1 activity in the adult neuron. This pathway constitutes a molecular link between aberrant cell-cycle activity and DNA damage and is a potential target for therapeutics against diseases and conditions involving neuronal death.
Figures





Comment in
-
A sequel to the tale of p25/Cdk5 in neurodegeneration.Neuron. 2008 Dec 10;60(5):731-2. doi: 10.1016/j.neuron.2008.11.020. Neuron. 2008. PMID: 19081365 Review.
References
-
- Adamec E, Vonsattel JP, Nixon RA. DNA strand breaks in Alzheimer’s disease. Brain Res. 1999;849:67–77. - PubMed
-
- Bates GP. Huntington’s disease. Exploiting expression. Nature. 2001;413:691, 693–694. - PubMed
-
- Boutillier AL, Trinh E, Loeffler JP. Constitutive repression of E2F1 transcriptional activity through HDAC proteins is essential for neuronal survival. Ann N Y Acad Sci. 2002;973:438–442. - PubMed
-
- Boutillier AL, Trinh E, Loeffler JP. Selective E2F-dependent gene transcription is controlled by histone deacetylase activity during neuronal apoptosis. J Neurochem. 2003;84:814–828. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

