Rationale and design of a trial of angiotensin-converting enzyme inhibition in infants with single ventricle
- PMID: 19081394
- PMCID: PMC2637409
- DOI: 10.1016/j.ahj.2008.08.030
Rationale and design of a trial of angiotensin-converting enzyme inhibition in infants with single ventricle
Abstract
Background: Angiotensin converting enzyme (ACE) inhibitors are known to improve clinical outcome and ventricular function in adults with heart failure. Infants with single-ventricle physiology show abnormalities in ventricular function as well as poor growth. The ability of an ACE inhibitor to preserve ventricular function and improve growth in these infants is unknown.
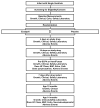
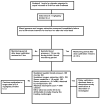
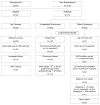
Methods: The Pediatric Heart Network designed a randomized, double-blind trial to compare outcomes in infants with single-ventricle physiology receiving enalapril or placebo. Neonates < or =45 days old were eligible. The primary outcome is weight-for-age Z-score at 14 months of age. Secondary outcomes include other measures of somatic growth, laboratory and functional measures of heart failure, developmental indices, measures of ventricular size and function, and the relationship of the renin-angiotensin-aldosterone system genotype to the response to enalapril. The incidence and spectrum of adverse events will also be compared between treatment groups.
Results: A total of 1,245 neonates were screened and 533 (43%) were eligible. The consent rate was 43%; 230 subjects were enrolled. Parental reluctance to participate was the primary reason for non-consent in 79% of the eligible nonconsenting patients. Randomized patients were older, more likely to be male, and more likely to have hypoplastic left heart syndrome than the eligible patients who did not enroll.
Conclusions: The results of this randomized trial will make an important contribution to the management of infants with single-ventricle physiology by determining whether initiation of ACE inhibition therapy in the neonatal period improves growth, clinical outcome, and ventricular function.
Trial registration: ClinicalTrials.gov NCT00113087.
Figures
References
-
- Leitch CA. Growth, nutrition and energy expenditure in pediatric heart failure. Prog in Ped Cardiology. 2000;11:195–202. - PubMed
-
- Cameron JW, Rosenthal A, Olson AD. Malnutrition in hospitalized children with congenital heart disease. Arch Pediatr Adolesc Med. 1995;149:1098–1102. - PubMed
-
- Cohen MI, Bush DM, Perry RJ, Spray TJ, Moshang T, Wernovsky G, et al. Somatic growth failure after the Fontan operation. Cardiol Young. 2002;10:447–457. - PubMed
-
- Day RW, Denton DM, Jackson WD. Growth of children with a functionally single ventricle following palliation at moderately increased altitude. Cardiol Young. 2000;10:193–200. - PubMed
-
- Vogt KN, Manlhiot C, Van Arsdell G, Russell JL, Mital S, McCrindle BW. Somatic growth in children with single ventricle physiology impact of physiologic state. J Am Coll Cardiol. 2007;50(19):1876–83. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

