A microwave assisted intramolecular-furan-Diels-Alder approach to 4-substituted indoles
- PMID: 19082013
- PMCID: PMC2736609
- DOI: 10.1039/b816989f
A microwave assisted intramolecular-furan-Diels-Alder approach to 4-substituted indoles
Abstract
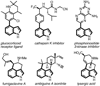
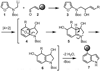
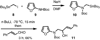
The key steps of a versatile new protocol for the convergent synthesis of 3,4-disubstituted indoles are the addition of an alpha-lithiated alkylaminofuran to a carbonyl compound, a microwave-accelerated intramolecular Diels-Alder cycloaddition and an in situ double aromatization reaction.
Figures
References
-
- Roach SL, Higuchi RI, Adams ME, Liu Y, Karanewsky DS, Marschke KB, Mais DE, Miner JN, Zhi L. Bioorg. Med. Chem. Lett. 2008;18:3504. - PubMed
- Li CS, Deschenes D, Desmarais S, Falgueyret J-P, Gauthier JY, Kimmel DB, Leger S, Masse F, McGrath ME, McKay DJ, Percival MD, Riendeau D, Rodan SB, Therien M, Truong V-L, Wesolowski G, Zamboni R, Black WC. Bioorg. Med. Chem. Lett. 2006;16:1985. - PubMed
- Doukas J, Wrasidlo W, Noronha G, Dneprovskaia E, Fine R, Weis S, Hood J, DeMaria A, Soll R, Cheresh D. Proc. Natl. Acad. Sci. U. S. A. 2006;103:19866. - PMC - PubMed
- Vinokurova NG, Boichenko DM, Baskunov BP, Zelenkova NF, Vepritskaya IG, Arinbasarov MU, Reshetilova TA. Appl. Biochem. Microbiol. 2001;37:184. - PubMed
- Huber U, Moore RE, Patterson GML. J. Nat. Prod. 1998;61:1304. - PubMed
- Smith S, Timmis GM. Nature. 1934;133:579.
-
- Glennon RA. J. Med. Chem. 1987;30:1. - PubMed
- Hugel HM, Kennaway D. Org. Prep. Proced. Int. 1995;27:1.
- Lounasmaa M, Tolvanen A. Nat. Prod. Rep. 2000;17:175. - PubMed
- Somei M, Yamada F. Nat. Prod. Rep. 2004;21:278. - PubMed
- Kawasaki T, Higuchi K. Nat. Prod. Rep. 2005;22:761. - PubMed
- O’Connor SE, Maresh JJ. Nat. Prod. Rep. 2006;23:532. - PubMed
-
-
For our previous studies on indoline and isoindolinone synthesis using nucleophilic addition and radical annulation strategies, see: Wipf P, Kim Y. Tetrahedron Lett. 1992;33:5477. Pierce JG, Waller DL, Wipf P. J. Organomet. Chem. 2007;692:4618. Wipf P, Maciejewski JP. Org. Lett. 2008;10:4383. Pierce JG, Kasi D, Fushimi M, Cuzzupe A, Wipf P. J. Org. Chem. 2008;73:7807..
-
-
- Kappe CO, Murphree SS, Padwa A. Tetrahedron. 1997;53:14179.
- Padwa A, Brodney MA, Dimitro M. J. Org. Chem. 1998;63:5304.
- Padwa A, Brodney MA, Liu Liu, Satake K, Wu T. J. Org. Chem. 1999;64:3595. - PubMed
- Padwa A, Brodney MA, Satake K, Straub CS. J. Org. Chem. 1999;64:4617. - PubMed
- Padwa A, Brodney MA, Lynch SM. J. Org. Chem. 2001;66:1716. - PubMed
- Bur SK, Lynch SM, Padwa A. Org. Lett. 2002;4:473. - PubMed
- Ginn JD, Padwa A. Org. Lett. 2002;4:1515. - PubMed
- Zhang H, Boonsombat J, Padwa A. Org. Lett. 2007;9:279. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources