Toxicokinetics and toxicodynamics of paraquat accumulation in mouse brain
- PMID: 19084006
- PMCID: PMC4016858
- DOI: 10.1016/j.expneurol.2008.11.003
Toxicokinetics and toxicodynamics of paraquat accumulation in mouse brain
Abstract
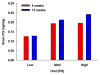
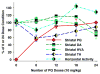
Paraquat (PQ) is a potential human neurotoxicant and is used in models of oxidative stress. We determined the toxicokinetics (TK) and toxicodynamics (TD) of PQ in adult mouse brain following repeated or prolonged PQ exposure. PQ accumulated in different brain regions and reached a plateau after approximately 18 i.p. (10 mg/kg) doses and resulted in modest morbidity and mortality unpredictably associated with dose interval and number. PQ had divergent effects on horizontal locomotor behavior depending on the number of doses. PQ decreased striatal dopamine levels after the 18th to 36th i.p. dose (10 mg/kg) and reduced the striatal level of tyrosine hydroxylase. Drinking water exposure to PQ (0.03- 0.05 mg/ml) did not result in any mortality and resulted in concentration and time dependent levels in the brain. The brain half-life of PQ varied with mouse strain. PQ accumulates and may saturate a site in mouse brain resulting in complex PQ level and duration-related consequences. These findings should alter our risk assessment of this compound and demonstrate a useful, but complex dynamic model for understanding the consequences of PQ in the brain.
Conflict of interest statement
There were no competing financial interests in this work.
Figures
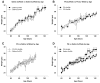

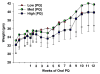
 ) PQ was measured in striatal samples after 6 (n = 4), 12 (n = 4), 18 (n = 4), or 24 (n = 8) (10 mg/kg) doses. There were no differences between the levels of PQ measured after dosing 2×or 3×/week and were combined. In Experiment II, the PQ level in striatum (n = 4,
) PQ was measured in striatal samples after 6 (n = 4), 12 (n = 4), 18 (n = 4), or 24 (n = 8) (10 mg/kg) doses. There were no differences between the levels of PQ measured after dosing 2×or 3×/week and were combined. In Experiment II, the PQ level in striatum (n = 4,
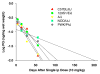
 ) was measured after a total of 7, 12, 18, 24, 30, and 36 (10 mg/kg) doses. PQ was also measured after the indicated number of doses from frontal cortex (n = 2,
) was measured after a total of 7, 12, 18, 24, 30, and 36 (10 mg/kg) doses. PQ was also measured after the indicated number of doses from frontal cortex (n = 2,
 ), hippocampus (n = 4,
), hippocampus (n = 4,
 ), and cerebellum (n = 4,
), and cerebellum (n = 4,
 ). Symbols without an error bar are the population mean and error bars indicate the s.e.m. Samples from the striatum in Experiments I and II, frontal cortex, cerebellum, and hippocampus were extracted and analyzed at different times.
). Symbols without an error bar are the population mean and error bars indicate the s.e.m. Samples from the striatum in Experiments I and II, frontal cortex, cerebellum, and hippocampus were extracted and analyzed at different times.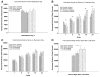
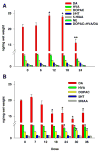
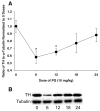
References
-
- Barlow BK, Bennice L, Thiruchelvam MJ, Cory-Slechta DA, Ballatori N, Richfield EK. Selective dithiocarbamates increase synaptosomal dopamine content, brain concentration of paraquat and correlate with potentiation of MPTP and paraquat toxicity. J Neurochem. 2003;85:1075–1086. - PubMed
-
- Barlow BK, Kochar J, Prasad K, Buckley B, Mirochnitchenko O, Richfield EK, Thiruchelvam M. Paraquat exposure during periods of neurodevelopment and the Parkinson’s disease phenotype. Soc Neurosci Abstract Poster # 25417/M10; San Diego, CA. Session # 254, Parkinson’s Disease Models and Mechanisms II.2007.
-
- Battaglia G, Busceti CL, Pontarelli F, Biagioni F, Fornai F, Paparelli A, Brunio V, Ruggieri S, Nicolett F. Protective role of group-II metabotropic glutamate receptors against nigro-striatal degeneration induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. Neuropharmacology. 2003;45:155–166. - PubMed
-
- Bowyer FJ, Tank AW, Newport GD, Slokker W, Jr, Ali SF, Holson RR. The influence of environmental temperature on the transient effects of methamphetamine on dopamine levels and dopamine release in rat striatum. J Pharmacol Exp Ther. 1992;260(2):817–824. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

