The intimate and mysterious relationship between proton channels and NADPH oxidase
- PMID: 19084015
- PMCID: PMC2630394
- DOI: 10.1016/j.febslet.2008.12.005
The intimate and mysterious relationship between proton channels and NADPH oxidase
Abstract
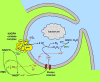
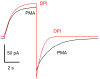
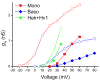
Voltage gated proton channels and NADPH oxidase function cooperatively in phagocytes during the respiratory burst, when reactive oxygen species are produced to kill microbial invaders. Although these molecules are distinct entities, with no proven physical interaction, their presence and activity in many cells appears to be coordinated. We describe these interactions and discuss several types of mechanisms that might explain them.
Figures
References
-
- DeCoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83:475–579. - PubMed
-
- Mahaut-Smith M. The effect of zinc on calcium and hydrogen ion currents in intact snail neurones. J Exp Biol. 1989;145:455–64. - PubMed
-
- DeCoursey TE, Cherny VV. Pharmacology of voltage-gated proton channels. Curr Pharm Des. 2007;13:2406–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

