Role of late sodium current in modulating the proarrhythmic and antiarrhythmic effects of quinidine
- PMID: 19084812
- PMCID: PMC2669543
- DOI: 10.1016/j.hrthm.2008.09.008
Role of late sodium current in modulating the proarrhythmic and antiarrhythmic effects of quinidine
Abstract
Background: Quinidine is used to treat atrial fibrillation and ventricular arrhythmias. However, at low concentrations, it can induce torsade de pointes (TdP).
Objective: The purpose of this study was to examine the role of late sodium current (I(Na)) as a modulator of the arrhythmogenicity of quinidine in female rabbit isolated hearts and cardiomyocytes.
Methods: Epicardial and endocardial monophasic action potentials (MAPs), ECG signals, and ion channel currents were measured. The sea anemone toxin ATX-II was used to increase late I(Na).
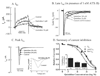
Results: Quinidine had concentration-dependent and often biphasic effects on measures of arrhythmogenicity. Quinidine increased the duration of epicardial MAP (MAPD(90)), QT interval, transmural dispersion of repolarization (TDR), and ventricular effective refractory period. Beat-to-beat variability of MAPD(90) (BVR), the interval from peak to end of the T wave (Tpeak-Tend) and index of Tpeak-Tend/QT interval were greater at 0.1 to 3 micromol/L than at 10-30 micromol/L quinidine. In the presence of 1 nmol/L ATX-II, quinidine caused significantly greater concentration-dependent and biphasic changes of Tpeak-Tend, TDR, BVR, and index of Tpeak-Tend/QT interval. Quinidine (1 micromol/L) induced TdP in 2 and 13 of 14 hearts in the absence and presence of ATX-II, respectively. Increases of BVR, index of Tpeak-Tend/QT interval, and Tpeak-Tend were associated with quinidine-induced TdP. Quinidine inhibited I(Kr), peak I(Na), and late I(Na) with IC(50)s of 4.5 +/- 0.3 micromol/L, 11.0 +/- 0.7 micromol/L, and 12.0 +/- 0.7 micromol/L.
Conclusion: Quinidine had biphasic proarrhythmic effects in the presence of ATX-II, suggesting that late I(Na) is a modulator of the arrhythmogenicity of quinidine. Enhancement of late I(Na) increased proarrhythmia caused by low but not high concentrations of quinidine.
Figures




References
-
- Grace AA, Camm AJ. Quinidine. N Engl J Med. 1998;338:35–45. - PubMed
-
- Iost N, Virag L, Varro A, et al. Comparison of the effect of class IA antiarrhythmic drugs on transmembrane potassium currents in rabbit ventricular myocytes. J Cardiovasc Pharmacol Ther. 2003;8:31–41. - PubMed
-
- Kaufman ES. Quinidine in short QT syndrome: an old drug for a new disease. J Cardiovasc Electrophysiol. 2007;18:665–666. - PubMed
-
- Milberg P, Tegelkamp R, Osada N, et al. Reduction of dispersion of repolarization and prolongation of postrepolarization refractoriness explain the antiarrhythmic effects of quinidine in a model of short QT syndrome. J Cardiovasc Electrophysiol. 2007;18:658–664. - PubMed
-
- Marquez MF, Salica G, Hermosillo AG, et al. Ionic basis of pharmacological therapy in Brugada syndrome. J Cardiovasc Electrophysiol. 2007;18:234–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

