Vitamin E treatment for children with chronic hepatitis B: a randomized placebo controlled trial
- PMID: 19084935
- PMCID: PMC2776878
- DOI: 10.3748/wjg.14.7208
Vitamin E treatment for children with chronic hepatitis B: a randomized placebo controlled trial
Abstract
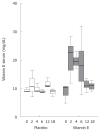
Aim: To evaluate the safety and efficacy of Vitamin E in children with chronic hepatitis B.
Methods: We randomly assigned patients with chronic hepatitis B, positive for hepatitis B e antigen (HBeAg), to receive either Vitamin E or placebo once daily for 6 mo in a 3:1 ratio and double-blind manner. The primary end point was HBeAg seroconversion, defined as the loss of HBeAg, undetectable levels of serum hepatitis B virus DNA, and the appearance of antibodies against HBeAg 12 mo after therapy.
Results: At baseline visit, 49 patients had normal and 43 had increased serum aminotransferase levels. Twenty-nine patients did not respond to previous treatment with interferon-alpha or lamivudine. Seventy-six children completed the study; 16 were non-compliant (n = 7), lost to follow-up (n = 7), or started another antiviral treatment (n = 3). Intention-to-treat analysis showed HBeAg seroconversion in 16 children (23.2%) treated with Vitamin E and two (8.7%) in the placebo group (P = 0.13). Vitamin E was well tolerated.
Conclusion: There is only a tendency that Vitamin E may promote HBeAg seroconversion. Therefore larger studies are needed to clarify the role of antioxidants in the therapy of chronic hepatitis B.
Figures
References
-
- Hahne S, Ramsay M, Balogun K, Edmunds WJ, Mortimer P. Incidence and routes of transmission of hepatitis B virus in England and Wales, 1995-2000: implications for immunisation policy. J Clin Virol. 2004;29:211–220. - PubMed
-
- Lai CL, Lok AS, Lin HJ, Wu PC, Yeoh EK, Yeung CY. Placebo-controlled trial of recombinant alpha 2-interferon in Chinese HBsAg-carrier children. Lancet. 1987;2:877–880. - PubMed
-
- Hui CK, Leung N, Shek TW, Yao H, Lee WK, Lai JY, Lai ST, Wong WM, Lai LS, Poon RT, et al. Sustained disease remission after spontaneous HBeAg seroconversion is associated with reduction in fibrosis progression in chronic hepatitis B Chinese patients. Hepatology. 2007;46:690–698. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical