Three-dimensional tissue models of normal and diseased skin
- PMID: 19085986
- PMCID: PMC2811850
- DOI: 10.1002/0471143030.cb1909s41
Three-dimensional tissue models of normal and diseased skin
Abstract
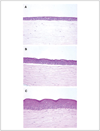
Over the last decade, the development of in vitro, human, three-dimensional (3D) tissue models, known as human skin equivalents (HSEs), has furthered understanding of epidermal cell biology and provided novel experimental systems. Signaling pathways that mediate the linkage between growth and differentiation function optimally when cells are spatially organized to display the architectural features seen in vivo, but are uncoupled and lost in two-dimensional culture systems. HSEs consist of a stratified squamous epithelium grown at an air-liquid interface on a collagen matrix populated with dermal fibroblasts. These 3D tissues demonstrate in vivo-like epithelial differentiation and morphology, and rates of cell division, similar to those found in human skin. This unit describes fabrication of HSEs, allowing the generation of human tissues that mimic the morphology, differentiation, and growth of human skin, as well as disease processes of cancer and wound re-epithelialization, providing powerful new tools for the study of diseases in humans.
Copyright 2008 by John Wiley & Sons, Inc.
Figures


References
-
- Andriani F, Margulis A, Lin N, Griffey S, Garlick JA. Analysis of microenvironmental factors contributing to basement membrane assembly and normalized epidermal phenotype. J. Invest. Dermatol. 2003;120:923–931. - PubMed
-
- Andriani F, Garfield J, Fusenig NE, Garlick JA. Basement membrane proteins promote progression of intraepithelial neoplasia in 3-dimensional models of human stratified epithelium. Int. J. Cancer. 2004;108:348–357. - PubMed
-
- Garlick JA, Taichman LB. Effect of TGF-beta 1 on re-epithelialization of human keratinocytes in vitro: An organotypic model. J. Invest. Dermatol. 1994a;103:554–559. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

