Loss of Notch signalling induced by Dll4 causes arterial calibre reduction by increasing endothelial cell response to angiogenic stimuli
- PMID: 19087347
- PMCID: PMC2637862
- DOI: 10.1186/1471-213X-8-117
Loss of Notch signalling induced by Dll4 causes arterial calibre reduction by increasing endothelial cell response to angiogenic stimuli
Abstract
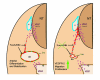
Background: In the vascular system, Notch receptors and ligands are expressed mainly on arteries, with Delta-like 4 (Dll4) being the only ligand known to be expressed early during the development of arterial endothelial cells and capillaries. Dll4 null embryos die very early in development with severely reduced arterial calibre and lumen and loss of arterial cell identity.
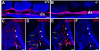
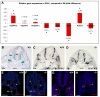
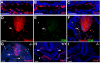
Results: The current detailed analysis of these mutants shows that the arterial defect precedes the initiation of blood flow and that the arterial Dll4-/- endothelial cells proliferate and migrate more actively. Dll4-/- mutants reveal a defective basement membrane around the forming aorta and increased endothelial cell migration from the dorsal aorta to peripheral regions, which constitute the main causes of arterial lumen reduction in these embryos. The increased proliferation and migration of Dll4-/- endothelial cells was found to coincide with increased expression of the receptors VEGFR-2 and Robo4 and with downregulation of the TGF-beta accessory receptor Endoglin.
Conclusion: Together, these results strongly suggest that Notch signalling can increase arterial stability and calibre by decreasing the response of arterial endothelial cells to local gradients of pro-angiogenic factors like VEGF.
Figures



References
-
- Fong GH, Zhang L, Bryce DM, Peng J. Increased hemangioblast commitment, not vascular disorganization, is the primary defect in flt-1 knock-out mice. Development. 1999;126:3015–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

