Advances in breast cancer: pathways to personalized medicine
- PMID: 19088015
- PMCID: PMC4535810
- DOI: 10.1158/1078-0432.CCR-08-1211
Advances in breast cancer: pathways to personalized medicine
Abstract
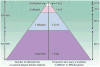
Breast cancer is a complex disease caused by the progressive accumulation of multiple gene mutations combined with epigenetic dysregulation of critical genes and protein pathways. There is substantial interindividual variability in both the age at diagnosis and phenotypic expression of the disease. With an estimated 1,152,161 new breast cancer cases diagnosed worldwide per year, cancer control efforts in the postgenome era should be focused at both population and individual levels to develop novel risk assessment and treatment strategies that will further reduce the morbidity and mortality associated with the disease. The discovery that mutations in the BRCA1 and BRCA2 genes increase the risk of breast and ovarian cancers has radically transformed our understanding of the genetic basis of breast cancer, leading to improved management of high-risk women. A better understanding of tumor host biology has led to improvements in the multidisciplinary management of breast cancer, and traditional pathologic evaluation is being complemented by more sophisticated genomic approaches. A number of genomic biomarkers have been developed for clinical use, and increasingly, pharmacogenetic end points are being incorporated into clinical trial design. For women diagnosed with breast cancer, prognostic or predictive information is most useful when coupled with targeted therapeutic approaches, very few of which exist for women with triple-negative breast cancer or those with tumors resistant to chemotherapy. The immediate challenge is to learn how to use the molecular characteristics of an individual and their tumor to improve detection and treatment, and ultimately to prevent the development of breast cancer. The five articles in this edition of CCR Focus highlight recent advances and future directions on the pathway to individualized approaches for the early detection, treatment, and prevention of breast cancer.
Conflict of interest statement
Figures


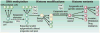
References
-
- Schubert EL, Mefford HC, Dann JL, Argonza RH, Hull J, King MC. BRCA1 and BRCA2 mutations in Ashkenazi Jewish families with breast and ovarian cancer. Genet Test. 1997;1:41–6. - PubMed
-
- Fackenthal JD, Olopade OI. Breast cancer risk associated with BRCA1 and BRCA2 in diverse populations. Nat Rev Cancer. 2007;7:937–48. - PubMed
-
- John EM, Miron A, Gong G, et al. Prevalence of pathogenic BRCA1 mutation carriers in 5 US racial/ethnic groups. JAMA. 2007;298:2869–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

