Phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with metastatic melanoma
- PMID: 19088048
- PMCID: PMC2629404
- DOI: 10.1158/1078-0432.CCR-08-1002
Phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with metastatic melanoma
Abstract
Purpose: Activation of the mitogen-activated protein kinase (MAPK) pathway and the phosphatidylinositol 3-kinase/AKT pathway seems to be critical for melanoma proliferation. Components of these pathways are client proteins of heat-shock protein 90 (hsp90), suggesting that inhibition of hsp90 could have significant antimelanoma effects. We conducted a phase II trial using the hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin (17-AAG) in melanoma patients. The primary end points were clinical responses and whether treatment inhibited MAPK pathway activity.
Experimental design: Melanoma patients with measurable disease were stratified on the basis of whether or not their tumor harbored a V600E BRAF mutation. The hsp90 inhibitor 17-AAG was administered i.v. once weekly x 6 weeks at 450 mg/m2. Tumor biopsies were obtained pretreatment and 18 to 50 hours after the first dose of 17-AAG, and were snap-frozen.
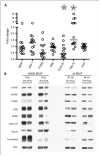
Results: Fifteen evaluable patients were treated; nine had BRAF mutations and six were wild-type. No objective responses were observed. Western blot analysis of tumor biopsies showed an increase in hsp70 and a decrease in cyclin D1 expression in the posttreatment biopsies but no significant effect on RAF kinases or phospho-extracellular signal-regulated kinase expression. Plasma analyzed by mutant-specific PCR for V600E BRAF showed 86% sensitivity and 67% specificity in predicting tumor DNA sequencing results.
Conclusions: At this dose and schedule of 17-AAG, the effects of 17-AAG on RAF kinase expression were short-lived, and no objective antimelanoma responses were seen. Future trials in melanoma should focus on a more potent hsp90 inhibitor or a formulation that can be administered chronically for a more prolonged suppression of the MAPK pathway.
Figures
References
-
- Fecher LA, Cummings SD, Keefe MJ, Alani RM. Toward a molecular classification of melanoma. J Clin Oncol. 2007;25:1606–20. - PubMed
-
- Kalinsky K, Haluska FG. Novel inhibitors in the treatment of metastatic melanoma. Expert Rev Anticancer Ther. 2007;7:715–24. - PubMed
-
- Johansson P, Pavey S, Hayward N. Confirmation of a BRAF mutation-associated gene expression signature in melanoma. Pigment Cell Res. 2007;20:216–21. - PubMed
-
- Goel VK, Lazar AJ, Warneke CL, Redston MS, Haluska FG. Examination of mutations in BRAF, NRAS, PTEN in primary cutaneous melanoma. J Invest Dermatol. 2006;126:154–60. - PubMed
-
- Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell. 1997;89:239–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

