Embryonic caffeine exposure induces adverse effects in adulthood
- PMID: 19088180
- PMCID: PMC2660649
- DOI: 10.1096/fj.08-124941
Embryonic caffeine exposure induces adverse effects in adulthood
Abstract
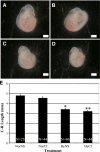
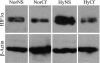
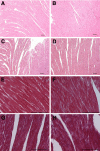
The purpose of this study was to determine both the short-term effects on cardiac development and embryo growth and the long-term effects on cardiac function and body composition of in utero caffeine exposure. Pregnant mice (C57BL/6) were exposed to hypoxia (10% O(2)) or room air from embryonic days (E) 8.5-10.5, and treated with caffeine (20 mg/kg, i.p.) or vehicle (normal saline, 0.9% NaCl). This caffeine dose results in a circulating level that is equivalent to 2 cups of coffee in humans. Hypoxic exposure acutely reduced embryonic growth by 30%. Exposure to a single dose of caffeine inhibited cardiac ventricular development by 53% in hypoxia and 37% in room air. Caffeine exposure resulted in inhibition of hypoxia-induced HIF1alpha protein expression in embryos by 40%. When offspring from dams treated with a single dose of caffeine were studied in adulthood, we observed that caffeine treatment alone resulted in a decrease in cardiac function of 38%, as assessed by echocardiography. We also observed a 20% increase in body fat with male mice exposed to caffeine. Caffeine was dissolved in normal saline, so it was used as a control. Room air controls were used to compare to the hypoxic mice. Exposure to a single dose of caffeine during embryogenesis results in both short-term effects on cardiac development and long-term effects on cardiac function.
Figures


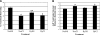
Similar articles
-
Embryonic caffeine exposure acts via A1 adenosine receptors to alter adult cardiac function and DNA methylation in mice.PLoS One. 2014 Jan 27;9(1):e87547. doi: 10.1371/journal.pone.0087547. eCollection 2014. PLoS One. 2014. PMID: 24475304 Free PMC article.
-
Modest maternal caffeine exposure affects developing embryonic cardiovascular function and growth.Am J Physiol Heart Circ Physiol. 2008 May;294(5):H2248-56. doi: 10.1152/ajpheart.91469.2007. Epub 2008 Mar 21. Am J Physiol Heart Circ Physiol. 2008. PMID: 18359892
-
Alteration of effective exposure of dam and embryo to caffeine and its metabolites by treatment of mice with beta-naphthoflavone.Toxicol Appl Pharmacol. 1987 Apr;88(2):282-93. doi: 10.1016/0041-008x(87)90013-5. Toxicol Appl Pharmacol. 1987. PMID: 3564044
-
Long-term consequences of disrupting adenosine signaling during embryonic development.Mol Aspects Med. 2017 Jun;55:110-117. doi: 10.1016/j.mam.2017.02.001. Epub 2017 Feb 13. Mol Aspects Med. 2017. PMID: 28202385 Free PMC article. Review.
-
Methylxanthines during pregnancy and early postnatal life.Handb Exp Pharmacol. 2011;(200):373-89. doi: 10.1007/978-3-642-13443-2_14. Handb Exp Pharmacol. 2011. PMID: 20859804 Review.
Cited by
-
Effect of Caffeine Chronically Consumed During Pregnancy on Adenosine A1 and A2A Receptors Signaling in Both Maternal and Fetal Heart from Wistar Rats.J Caffeine Res. 2014 Dec 1;4(4):115-126. doi: 10.1089/jcr.2014.0010. J Caffeine Res. 2014. PMID: 25538864 Free PMC article.
-
In utero exposure to diesel exhaust air pollution promotes adverse intrauterine conditions, resulting in weight gain, altered blood pressure, and increased susceptibility to heart failure in adult mice.PLoS One. 2014 Feb 12;9(2):e88582. doi: 10.1371/journal.pone.0088582. eCollection 2014. PLoS One. 2014. PMID: 24533117 Free PMC article.
-
Caffeine exposure alters cardiac gene expression in embryonic cardiomyocytes.Am J Physiol Regul Integr Comp Physiol. 2014 Dec 15;307(12):R1471-87. doi: 10.1152/ajpregu.00307.2014. Epub 2014 Oct 29. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 25354728 Free PMC article.
-
Using Zebrafish to Implement a Course-Based Undergraduate Research Experience to Study Teratogenesis in Two Biology Laboratory Courses.Zebrafish. 2016 Aug;13(4):293-304. doi: 10.1089/zeb.2015.1107. Epub 2016 Feb 1. Zebrafish. 2016. PMID: 26829498 Free PMC article.
-
Caffeine acts via A1 adenosine receptors to disrupt embryonic cardiac function.PLoS One. 2011;6(12):e28296. doi: 10.1371/journal.pone.0028296. Epub 2011 Dec 2. PLoS One. 2011. PMID: 22164264 Free PMC article.
References
-
- Ijzerman A, van Rhee A M. Modulators of adenosine uptake, release, and inactivation. Jacobson K A, Jarvis M F, editors. New York: Wiley-Liss; Purinergic Approaches in Experimental Therapeutics. 1997:129–148.
-
- Koos B J, Mason B A, Ervin M G. Adenosine mediates hypoxic release of arginine vasopressin in fetal sheep. Am J Physiol. 1994;266:R215–R220. - PubMed
-
- Koos B J, Mason B A, Punla O, Adinolfi A M. Hypoxic inhibition of breathing in fetal sheep: relationship to brain adenosine concentrations. J Appl Physiol. 1994;77:2734–2739. - PubMed
-
- Yoneyama Y, Shin S, Iwasaki T, Power G G, Araki T. Relationship between plasma adenosine concentration and breathing movements in growth-retarded fetuses. Am J Obstet Gynecol. 1994;171:701–706. - PubMed
-
- Yoneyama Y, Wakatsuki M, Sawa R, Kamoi S, Takahashi H, Shin S, Kawamura T, Power G G, Araki T. Plasma adenosine concentration in appropriate- and small-for-gestational-age fetuses. Am J Obstet Gynecol. 1994;170:684–688. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

