In vivo imaging of murid herpesvirus-4 infection
- PMID: 19088269
- PMCID: PMC2885022
- DOI: 10.1099/vir.0.006569-0
In vivo imaging of murid herpesvirus-4 infection
Abstract
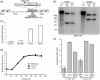
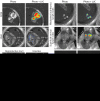
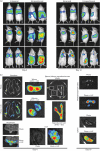
Luciferase-based imaging allows a global view of microbial pathogenesis. We applied this technique to gammaherpesvirus infection by inserting a luciferase expression cassette into the genome of murine herpesvirus-4 (MuHV-4). The recombinant virus strongly expressed luciferase in lytically infected cells without significant attenuation. We used it to compare different routes of virus inoculation. After intranasal infection of anaesthetized mice, luciferase was expressed in the nose and lungs for 7-10 days and in lymphoid tissue, most consistently the superficial cervical lymph nodes, for up to 30 days. Gastrointestinal infection was not observed. Intraperitoneal infection was very different to intranasal, with strong luciferase expression in the liver, kidneys, intestines, reproductive tract and spleen, but none in the nose or lungs. The nose has not previously been identified as a site of MuHV-4 infection. After intranasal infection of non-anaesthetized mice, it was the only site of non-lymphoid luciferase expression. Nevertheless, lymphoid colonization and persistence were still established, even at low inoculation doses. In contrast, virus delivered orally was very poorly infectious. Inoculation route therefore had a major impact on pathogenesis. Low dose intranasal infection without anaesthesia seems most likely to mimic natural transmission, and may therefore be particularly informative about normal viral gene functions.
Figures





Similar articles
-
Comparative study of murid gammaherpesvirus 4 infection in mice and in a natural host, bank voles.J Gen Virol. 2010 Oct;91(Pt 10):2553-63. doi: 10.1099/vir.0.023481-0. Epub 2010 Jun 9. J Gen Virol. 2010. PMID: 20538905
-
Murid herpesvirus-4 lacking thymidine kinase reveals route-dependent requirements for host colonization.J Gen Virol. 2009 Jun;90(Pt 6):1461-1470. doi: 10.1099/vir.0.010603-0. Epub 2009 Mar 4. J Gen Virol. 2009. PMID: 19264614 Free PMC article.
-
Experimental infection of laboratory-bred bank voles (Myodes glareolus) with murid herpesvirus 4.Arch Virol. 2012 Nov;157(11):2207-12. doi: 10.1007/s00705-012-1397-5. Epub 2012 Jul 11. Arch Virol. 2012. PMID: 22782137
-
Murid herpesvirus 4 (MuHV-4, prototype strain MHV-68) as an important model in global research of human oncogenic gammaherpesviruses.Acta Virol. 2020;64(2):167-176. doi: 10.4149/av_2020_206. Acta Virol. 2020. PMID: 32551785 Review.
-
Rhesus macaque rhadinovirus-associated disease.Curr Opin Virol. 2013 Jun;3(3):245-50. doi: 10.1016/j.coviro.2013.05.016. Epub 2013 Jun 6. Curr Opin Virol. 2013. PMID: 23747119 Free PMC article. Review.
Cited by
-
IFN-λ Decreases Murid Herpesvirus-4 Infection of the Olfactory Epithelium but Fails to Prevent Virus Reactivation in the Vaginal Mucosa.Viruses. 2019 Aug 16;11(8):757. doi: 10.3390/v11080757. Viruses. 2019. PMID: 31426334 Free PMC article.
-
Applications of in vivo imaging in the evaluation of the pathophysiology of viral and bacterial infections and in development of countermeasures to BSL3/4 pathogens.Mol Imaging Biol. 2015 Feb;17(1):4-17. doi: 10.1007/s11307-014-0759-7. Mol Imaging Biol. 2015. PMID: 25008802 Free PMC article. Review.
-
Conquering the Host: Determinants of Pathogenesis Learned from Murine Gammaherpesvirus 68.Annu Rev Virol. 2021 Sep 29;8(1):349-371. doi: 10.1146/annurev-virology-011921-082615. Annu Rev Virol. 2021. PMID: 34586873 Free PMC article.
-
An in vitro system for studying murid herpesvirus-4 latency and reactivation.PLoS One. 2010 Jun 11;5(6):e11080. doi: 10.1371/journal.pone.0011080. PLoS One. 2010. PMID: 20552028 Free PMC article.
-
Systemic and local infection routes govern different cellular dissemination pathways during gammaherpesvirus infection in vivo.J Virol. 2013 Apr;87(8):4596-608. doi: 10.1128/JVI.03135-12. Epub 2013 Feb 13. J Virol. 2013. PMID: 23408606 Free PMC article.
References
-
- Belz, G. T., Wilson, N. S., Kupresanin, F., Mount, A. M. & Smith, C. M. (2007). Shaping naive and memory CD8+ T cell responses in pathogen infections through antigen presentation. Adv Exp Med Biol 590, 31–42. - PubMed
-
- Blasdell, K., McCracken, C., Morris, A., Nash, A. A., Begon, M., Bennett, M. & Stewart, J. P. (2003). The wood mouse is a natural host for murid herpesvirus 4. J Gen Virol 84, 111–113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

