Efficient intrathymic gene transfer following in situ administration of a rAAV serotype 8 vector in mice and nonhuman primates
- PMID: 19088703
- PMCID: PMC2835094
- DOI: 10.1038/mt.2008.272
Efficient intrathymic gene transfer following in situ administration of a rAAV serotype 8 vector in mice and nonhuman primates
Abstract
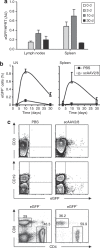
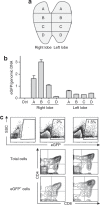
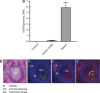
The thymus is the primary site of T-cell development and plays a key role in the induction of self-tolerance. We previously showed that the intrathymic (i.t.) injection of a transgene-expressing lentiviral vector (LV) in mice can result in the correction of a T cell-specific genetic defect. Nevertheless, the efficiency of thymocyte transduction did not exceed 0.1-0.3% and we were unable to detect any thymus transduction in macaques. As such, we initiated studies to assess the capacity of recombinant adeno-associated virus (rAAV) vectors to transduce murine and primate thymic cells. In vivo administration of AAV serotype 2-derived single-stranded AAV (ssAAV) and self-complementary AAV (scAAV) vectors pseudotyped with capsid proteins of serotypes 1, 2, 4, 5, and 8 demonstrated that murine thymus transduction was significantly enhanced by scAAV2/8. Transgene expression was detected in 5% of thymocytes and, notably, transduced cells represented 1% of peripheral T lymphocytes. Moreover, i.t. administration of scAAV2/8 particles in macaques, by endoscopic-mediated guidance, resulted in significant gene transfer. Thus, in healthy animals, where thymic gene transfer does not provide a selective advantage, scAAV2/8 is a unique tool promoting the in situ transduction of thymocytes with the subsequent export of gene-modified lymphocytes to the periphery.
Figures


Similar articles
-
Comparison of the ability of adeno-associated viral vectors pseudotyped with serotype 2, 5, and 8 capsid proteins to mediate efficient transduction of the liver in murine and nonhuman primate models.Mol Ther. 2005 Jun;11(6):875-88. doi: 10.1016/j.ymthe.2004.12.022. Mol Ther. 2005. PMID: 15922958
-
Intrathymic adeno-associated virus gene transfer rapidly restores thymic function and long-term persistence of gene-corrected T cells.J Allergy Clin Immunol. 2020 Feb;145(2):679-697.e5. doi: 10.1016/j.jaci.2019.08.029. Epub 2019 Sep 9. J Allergy Clin Immunol. 2020. PMID: 31513879 Free PMC article.
-
Assessment of ocular transduction using single-stranded and self-complementary recombinant adeno-associated virus serotype 2/8.Gene Ther. 2008 Mar;15(6):463-7. doi: 10.1038/sj.gt.3303074. Epub 2007 Nov 15. Gene Ther. 2008. PMID: 18004402
-
Expressing Transgenes That Exceed the Packaging Capacity of Adeno-Associated Virus Capsids.Hum Gene Ther Methods. 2016 Feb;27(1):1-12. doi: 10.1089/hgtb.2015.140. Hum Gene Ther Methods. 2016. PMID: 26757051 Free PMC article. Review.
-
The complex and evolving story of T cell activation to AAV vector-encoded transgene products.Mol Ther. 2011 Jan;19(1):16-27. doi: 10.1038/mt.2010.250. Epub 2010 Nov 30. Mol Ther. 2011. PMID: 21119617 Free PMC article. Review.
Cited by
-
DNA cleavage enzymes for treatment of persistent viral infections: recent advances and the pathway forward.Virology. 2014 Apr;454-455:353-61. doi: 10.1016/j.virol.2013.12.037. Epub 2014 Jan 31. Virology. 2014. PMID: 24485787 Free PMC article. Review.
-
Self-complementary adeno-associated viral vectors for gene therapy of hemophilia B: progress and challenges.Expert Rev Hematol. 2011 Oct;4(5):539-49. doi: 10.1586/ehm.11.48. Expert Rev Hematol. 2011. PMID: 21939421 Free PMC article. Review.
-
Intrathymic AAV delivery results in therapeutic site-specific integration at TCR loci in mice.Blood. 2023 May 11;141(19):2316-2329. doi: 10.1182/blood.2022017378. Blood. 2023. PMID: 36790505 Free PMC article.
-
Induction of immune tolerance to a therapeutic protein by intrathymic gene delivery.Mol Ther. 2010 Dec;18(12):2146-54. doi: 10.1038/mt.2010.164. Epub 2010 Jul 27. Mol Ther. 2010. PMID: 20664526 Free PMC article.
-
Molecular and cellular basis of T cell lineage commitment.Semin Immunol. 2010 Oct;22(5):270-5. doi: 10.1016/j.smim.2010.04.016. Epub 2010 Jul 13. Semin Immunol. 2010. PMID: 20630771 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

