Irinotecan plus carboplatin for patients with carcinoma of unknown primary site
- PMID: 19088717
- PMCID: PMC2634680
- DOI: 10.1038/sj.bjc.6604829
Irinotecan plus carboplatin for patients with carcinoma of unknown primary site
Abstract
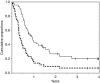
Carcinoma of unknown primary site (CUP) is rarely encountered in clinical practice and optimal chemotherapy has not yet been established. This phase II study was conducted to evaluate the efficacy and toxicity of combined irinotecan+carboplatin therapy in chemotherapy-naive patients with CUP. Irinotecan was administered at 60 mg m(-2) as a 90-min intravenous infusion on days 1, 8 and 15. Carboplatin was administered at an area-under-the curve of 5 mg ml(-1) min as a 60-min intravenous infusion on day 1. This cycle was repeated every 28 days for up to six cycles. Forty-five patients were enrolled in the study. An intent-to-treat analysis revealed an objective response rate to the treatment of 41.9% (95% confidence interval, 27.0-57.9%). The median time to progression was 4.8 months and the median survival was 12.2 months. The 1- and 2-year survival rates were 44 and 27%, respectively. The most frequent grade 3 or more severe adverse events were leukopaenia (21%), neutropaenia (33%), anaemia (25%) and thrombocytopaenia (20%). Thus, the combination of irinotecan plus carboplatin was found to be active in patients with CUP. Therefore, the regimen may be one of the potentially available chemotherapeutic options for community standard of care in patients with a good performance status.
Figures
References
-
- Balaña C, Manzano JL, Moreno I, Cirauqui B, Abad A, Font A, Mate JL, Rosell R (2003) A phase II study of cisplatin, etoposide and gemcitabine in an unfavourable group of patients with carcinoma of unknown primary site. Ann Oncol 4: 1425–1429 - PubMed
-
- Briasoulis E, Fountzilas G, Bamias A, Dimopoulis MA, Xiros N, Aravantinos G, Samantas E, Kalofonos H, Makatsoris T, Mylonakis N, Papakostas P, Skarlos D, Varthalitis I, Pavlidis N (2008a) Multicenter phase II trial of irinotecan plus oxaliplatin in patients with poor-prognosis cancer of unknown primary: a Hellenic cooperative oncology group study. Cancer Chemother Pharmacol 62: 277–284 - PubMed
-
- Briasoulis E, Kalofonos H, Bafaloukos D, Samantas E, Fountzilas G, Xiros N, Skarlos D, Christodoulou C, Kosmidis P, Pavlidis N (2000) Carboplatin plus paclitaxel in unknown primary carcinoma; a phase II Hellenic Cooperative Oncology Group Study. J Clin Oncol 18: 3101–3107 - PubMed
-
- Briasoulis E, Pavlidis N, Felip E (2008b) Cancers of unknown primary site: ESMO clinical recommendation for diagnosis, treatment and follow-up. Ann Oncol 19(Suppl 2): ii106–ii107 - PubMed
-
- Calvert AH, Newell DR, Gumbrell LA, O’Reilly S, Burnell M, Boxall FE, Siddik ZH, Judson IR, Gore ME, Wiltshaw E (1989) Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 7: 1748–1756 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources