Modulation of cGMP in heart failure: a new therapeutic paradigm
- PMID: 19089342
- PMCID: PMC3835600
- DOI: 10.1007/978-3-540-68964-5_21
Modulation of cGMP in heart failure: a new therapeutic paradigm
Abstract
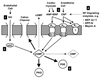
Heart failure (HF) is a common disease that continues to be associated with high morbidity and mortality warranting novel therapeutic strategies. Cyclic guanosine monophosphate (cGMP) is the second messenger of several important signaling pathways based on distinct guanylate cyclases (GCs) in the cardiovascular system. Both the nitric oxide/soluble GC (NO/sGC) as well as the natriuretic peptide/GC-A (NP/GC-A) systems are disordered in HF, providing a rationale for their therapeutic augmentation. Soluble GC activation with conventional nitrovasodilators has been used for more than a century but is associated with cGMP-independent actions and the development of tolerance, actions which novel NO-independent sGC activators now in clinical development lack. Activation of GC-A by administration of naturally occurring or designer natriuretic peptides is an emerging field, as is the inhibition of enzymes that degrade endogenous NPs. Finally, inhibition of cGMP-degrading phosphodiesterases, particularly phosphodiesterase 5 provides an additional strategy to augment cGMP-signaling.
Figures




References
-
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. - PubMed
-
- Bischoff E, Stasch JP. Effects of the sGC stimulator BAY 41-2272 are not mediated by phosphodiesterase 5 inhibition. Circulation. 2004;110:e320–e321. author reply e320–321. - PubMed
-
- Boerrigter G, Costello-Boerrigter LC, Cataliotti A, Lapp H, Stasch JP, Burnett JC., Jr Targeting heme-oxidized soluble guanylate cyclase in experimental heart failure. Hypertension. 2007;49:1128–1133. - PubMed
-
- Boerrigter G, Costello-Boerrigter LC, Cataliotti A, Tsuruda T, Harty GJ, Lapp H, et al. Cardiorenal and humoral properties of a novel direct soluble guanylate cyclase stimulator BAY 41–2272 in experimental congestive heart failure. Circulation. 2003;107:686–689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

