Isolation and culture of epithelial stem cells
- PMID: 19089359
- PMCID: PMC2760227
- DOI: 10.1007/978-1-59745-060-7_14
Isolation and culture of epithelial stem cells
Abstract
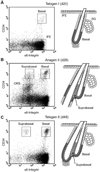
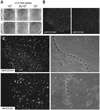
In the skin, epithelial stem cells in the hair follicle contribute not only to the generation of a new hair follicle with each hair cycle, but also to the repair of the epidermis during wound healing. When these stem cells are isolated and expanded in culture, they can give rise to hair follicles, sebaceous glands, and epidermis when combined with dermis and grafted back onto Nude mice. In this chapter, we provide a method for isolating hair follicle epithelial stem cells from the skin of adult mice using immunofluorescent labeling to allow for the specific purification of epithelial stem cells by fluorescence-activated cell sorting (FACS). Notably, this method relies exclusively on cell surface markers, making it suitable for use with any strain of mouse and at various stages of the hair cycle. We also provide a detailed protocol for culturing epithelial stem cells isolated by FACS, allowing for analysis using a wide variety of culture assays. Additionally, we provide notes on using cultured cells for specific applications, such as viral manipulation and grafting. These techniques should be useful for directly evaluating stem cell function in normal mice and in mice with skin defects.
Figures

References
-
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61(7):1329–1337. - PubMed
-
- Morris RJ, Potten CS. Highly persistent label-retaining cells in the hair follicles of mice and their fate following induction of anagen. J Invest Dermatol. 1999;112(4):470–475. - PubMed
-
- Taylor G, et al. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102(4):451–461. - PubMed
-
- Morris RJ, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22(4):411–417. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

