Inhibition of tumor-cell invasion with chlorotoxin-bound superparamagnetic nanoparticles
- PMID: 19089837
- PMCID: PMC2692352
- DOI: 10.1002/smll.200800646
Inhibition of tumor-cell invasion with chlorotoxin-bound superparamagnetic nanoparticles
Abstract
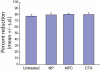
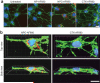
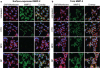
Nanoparticles have been investigated as drug delivery vehicles, contrast agents, and multifunctional devices for patient care. Current nanoparticle-based therapeutic strategies for cancer treatment are mainly based on delivery of chemotherapeutic agents to induce apoptosis or DNA/siRNA to regulate oncogene expression. Here, a nanoparticle system that demonstrates an alternative approach to the treatment of cancers through the inhibition of cell invasion, while serving as a magnetic resonance and optical imaging contrast agent, is presented. The nanoparticle comprises an iron oxide nanoparticle core conjugated with an amine-functionalized poly(ethylene glycol) silane and a small peptide, chlorotoxin (CTX), which enables the tumor cell-specific binding of the nanoparticle. It is shown that the nanoparticle exhibits substantially enhanced cellular uptake and an invasion inhibition rate of approximately 98% compared to unbound CTX ( approximately 45%). Significantly, the investigation from flow cytometry analysis, transmission electron microscopy, and fluorescent imaging reveals that the CTX-enabled nanoparticles deactivated the membrane-bound matrix metalloproteinase 2 (MMP-2) and induced increased internalization of lipid rafts that contain surface-expressed MMP-2 and volume-regulating ion channels through receptor-mediated endocytosis, leading to enhanced prohibitory effects. Since upregulation and activity of MMP-2 have been observed in tumors of neuroectodermal origin, and in cancers of the breast, colon, skin, lung, prostate, ovaries, and a host of others, this nanoparticle system can be potentially used for non-invasive diagnosis and treatment of a variety of cancer types.
Figures

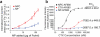


References
-
- Brigger I, Dubernet C, Couvreur P. Adv. Drug Delivery Rev. 2002;54:631–651. - PubMed
-
- Nie SM, Xing Y, Kim GJ, Simons JW. Annu. Rev. Biomed. Eng. 2007;9:257–288. - PubMed
-
- Zhao M, Beauregard DA, Loizou L, Davletov B, Brindle KM. Nat. Med. 2001;7:1241–1244. - PubMed
-
- Weissleder R, Moore A, Mahmood U, Bhorade R, Benveniste H, Chiocca EA, Basilion JP. Nat. Med. 2000;6:351–354. - PubMed
-
- Jun YW, Huh YM, Choi JS, Lee JH, Song HT, Kim S, Yoon S, Kim KS, Shin JS, Suh JS, Cheon J. J. Am. Chem. Soc. 2005;127:5732–5733. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

