Natural history of the E1-like superfamily: implication for adenylation, sulfur transfer, and ubiquitin conjugation
- PMID: 19089947
- PMCID: PMC2732565
- DOI: 10.1002/prot.22298
Natural history of the E1-like superfamily: implication for adenylation, sulfur transfer, and ubiquitin conjugation
Abstract
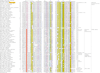
The E1-like superfamily is central to ubiquitin (Ub) conjugation, biosynthesis of cysteine, thiamine, and MoCo, and several secondary metabolites. Yet, its functional diversity and evolutionary history is not well understood. We develop a natural classification of this superfamily and use it to decipher the major adaptive trends occurring in the evolution of the E1-like superfamily. Within the Rossmann fold, E1-like proteins are closest to NAD(P)/FAD-dependent dehydrogenases and S-AdoMet-dependent methyltransferases. Hence, their phosphotransfer activity is an independent catalytic "invention" with respect to such activities seen in other Rossmannoid folds. Sequence and structure analysis reveals a striking diversity of residues and structures involved in adenylation, sulfotransfer, and substrate binding between different E1-like families, allowing us to predict previously uncharacterized functional adaptations. E1-like proteins are fused to several previously undetected domains, such as a predicted sulfur transfer domain containing a novel superfamily of the TATA-binding protein fold, different types of catalytic domains, a novel winged helix-turn-helix domain and potential adaptor domains related to Ub conjugation. On the basis of these fusions, we develop a generalized model for the linking of E1 catalyzed adenylation/thiolation with further downstream reactions. This is likely to involve a dynamic interplay between the E1 active sites and diverse fused C-terminal domains. We also predict participation of E1-like domains in previously uncharacterized bacterial secondary metabolism pathways, new cysteine biosynthesis systems, such as those associated with archaeal O-phosphoseryl tRNA, metal-sulfur cluster assembly (e.g., in nitrogen fixation) and Ub-conjugation. Evolutionary reconstructions suggest that the last universal common ancestor contained a single E1-like domain possessing both phosphotransfer and thiolating activities and participating in multiple sulfotransfer reactions. The E1-like superfamily subsequently expanded to include 26 families clustering into three major radiations. These are broadly involved in Ub activation, cofactor and cysteine biosynthesis, and biosynthesis of secondary metabolites. In light of this, we present evidence that in eukaryotes other E1-like enzymes such as Urm1 were independently recruited for Ubl conjugation, probably functioning without conventional E2-like enzymes.
Copyright 2008 Wiley-Liss, Inc.
Figures



References
-
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. New York, NY: Garland Science Publishing; 2002.
-
- Ciechanover A, Orian A, Schwartz AL. Ubiquitin-mediated proteolysis: biological regulation via destruction. Bioessays. 2000;22(5):442–451. - PubMed
-
- Duda DM, Walden H, Sfondouris J, Schulman BA. Structural analysis of Escherichia coli ThiF. J Mol Biol. 2005;349(4):774–786. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

