Tumor vasculature-targeted delivery of tumor necrosis factor-alpha
- PMID: 19090007
- PMCID: PMC8385542
- DOI: 10.1002/cncr.24001
Tumor vasculature-targeted delivery of tumor necrosis factor-alpha
Abstract
Background: Recently, considerable efforts have been directed toward antivascular therapy as a new modality to treat human cancers. However, targeting a therapeutic gene of interest to the tumor vasculature with minimal toxicity to other tissues remains the objective of antivascular gene therapy. Tumor necrosis factor-alpha (TNF-alpha) is a potent antivascular agent but has limited clinical utility because of significant systemic toxicity. At the maximum tolerated doses of systemic TNF-alpha, there is no meaningful antitumor activity. Hence, the objective of this study was to deliver TNF-alpha targeted to tumor vasculature by systemic delivery to examine its antitumor activity.
Methods: A hybrid adeno-associated virus phage vector (AAVP) was used that targets tumor endothelium to express TNF-alpha (AAVP-TNF-alpha). The activity of AAVP-TNF-alpha was analyzed in various in vitro and in vivo settings using a human melanoma tumor model.
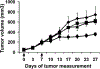
Results: In vitro, AAVP-TNF-alpha infection of human melanoma cells resulted in high levels of TNF-alpha expression. Systemic administration of targeted AAVP-TNF-alpha to melanoma xenografts in mice produced the specific delivery of virus to tumor vasculature. In contrast, the nontargeted vector did not target to tumor vasculature. Targeted AAVP delivery resulted in expression of TNF-alpha, induction of apoptosis in tumor vessels, and significant inhibition of tumor growth. No systemic toxicity to normal organs was observed.
Conclusions: Targeted AAVP vectors can be used to deliver TNF-alpha specifically to tumor vasculature, potentially reducing its systemic toxicity. Because TNF-alpha is a promising antivascular agent that currently is limited by its toxicity, the current results suggest the potential for clinical translation of this strategy.
Figures






References
-
- Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. - PubMed
-
- ten Hagen TL, Eggermont AM. Solid tumor therapy: manipulation of the vasculature with TNF. Technol Cancer Res Treat. 2003;2:195–203. - PubMed
-
- Scarpati EM, Sadler JE. Regulation of endothelial cell coagulant properties. Modulation of tissue factor, plasminogen activator inhibitors, and thrombomodulin by phorbol 12-myristate 13-acetate and tumor necrosis factor. J Biol Chem. 1989;264:20705–20713. - PubMed
-
- Friedl J, Puhlmann M, Bartlett DL, et al.Induction of permeability across endothelial cell monolayers by tumor necrosis factor (TNF) occurs via a tissue factor-dependent mechanism: relationship between the procoagulant and permeability effects of TNF. Blood. 2002;100:1334–1339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

