Efficacy, safety, and pharmacokinetics of candesartan cilexetil in hypertensive children aged 6 to 17 years
- PMID: 19090875
- PMCID: PMC8673170
- DOI: 10.1111/j.1751-7176.2008.00022.x
Efficacy, safety, and pharmacokinetics of candesartan cilexetil in hypertensive children aged 6 to 17 years
Abstract
This 4-week randomized, double blind, placebo-controlled study (N=240), 1-year open label trial (N=233), and single-dose pharmacokinetic study (N=22) evaluated candesartan cilexetil (3 doses) in hypertensive children aged 6 to 17 years. Seventy-one percent were 12 years of age or older, 71% were male, and 47% were black. Systolic (SBP)/diastolic (DBP) blood pressure declined 8.6/4.8-11.2/8.0 mm Hg with candesartan and 3.7/1.8 mm Hg with placebo (P<.01 compared to placebo for SBP and for the mid and high doses for DBP; placebo-corrected 4.9/3.0-7.5/6.2 mm Hg). The slopes for dose were not, however, different from zero (P>.05). The response rate (SBP and DBP <95th percentile) after 1 year was 53%. The pharmacokinetic profiles in 6- to 12- and 12- to 17-year-olds were similar and were comparable to adults. Eight candesartan patients discontinued treatment because of an adverse event. Candesartan is an effective, well-tolerated antihypertensive agent for children aged 6 to 17 years and has a pharmacokinetic profile that is similar to that in adults.
Figures

 Placebo,
Placebo,  Candesartan 2/4 mg.
Candesartan 2/4 mg.  Candesartan 8/16 mg.
Candesartan 8/16 mg.  Candesartan 16/32 mg.
Candesartan 16/32 mg.  All candesartan doses.
All candesartan doses.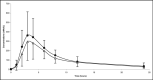
References
-
- Sorof JM, Lai D, Turner J, et al. Overweight, ethnicity, and the prevalence of hypertension in school‐aged children. Pediatrics. 2004;113:475–482. - PubMed
-
- The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. US Department of Health and Human Services . National Institute of Health; National Heart, Lung, and Blood Institute; 2005:1–48.
-
- Hanevold C, Waller J, Daniels S, et al. The effects of obesity, gender, and ethnic group on left ventricular hypertrophy and geometry in hypertensive children: a collaborative study of the International Pediatric Hypertension Association. Pediatrics. 2004;113:328–333. - PubMed
-
- Daniels SR, Loggie JM, Khoury P, et al. Left ventricular geometry and severe left ventricular hypertrophy in children and adolescents with essential hypertension. Circulation. 1998;97:1907–1911. - PubMed
-
- Brady TM, Fivush B, Flynn JT, et al. Ability of blood pressure to predict left ventricular hypertrophy in children with primary hypertension. J Pediatr. 2008;152:73–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

