Desmoglein 2 is a substrate of kallikrein 7 in pancreatic cancer
- PMID: 19091121
- PMCID: PMC2628383
- DOI: 10.1186/1471-2407-8-373
Desmoglein 2 is a substrate of kallikrein 7 in pancreatic cancer
Abstract
Background: In a previous report we have demonstrated that the chymotryptic-like serine protease kallikrein 7 (KLK7/hK7) is overexpressed in pancreatic cancer. In normal skin, hK7 is thought to participate in skin desquamation by contributing in the degradation of desmosomal components, such as desmogleins. Thus, the ability of hK7 to degrade desmogleins was assessed and the effect of hK7 expression on desmoglein 2 was examined in cultured pancreatic cancer cells.
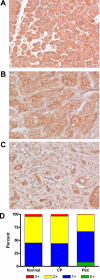
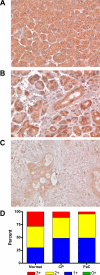
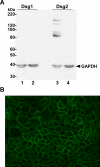
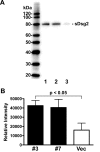
Methods: The expression of Dsg1, Dsg2, and Dsg3 in pancreatic tissues was examined by immunohistochemistry and their expression in two pancreatic cancer cell lines, BxPC-3 and Panc-1, was determined by western blot analysis. The ability of hK7 to degrade Dsg1 and Dsg2 was investigated using in vitro degradation assays. BxPC-3 cells stably transfected to overexpress hK7 were used to examine the effect of hK7 on cell-surface resident Dsg2.
Results: The levels of immunoreactive Dsg1 and Dsg2 were reduced in pancreatic adenocarcinomas compared with both normal pancreatic and chronic pancreatitis tissues. Among the desmosomal proteins examined, Dsg2 exhibited robust expression on the surface of BxPC-3 cells. When hK7 was overexpressed in this cell line, there was a significant increase in the amount of soluble Dsg2 released into the culture medium compared with vector-transfected control cells.
Conclusion: A reduction in the amount of the cell adhesion components Dsg1 and Dsg2 in pancreatic tumors suggests that loss of these desmosomal proteins may play a role in pancreatic cancer invasion. Using in vitro degradation assays, both Dsg1 and Dsg2 could be readily proteolyzed by hK7, which is overexpressed in pancreatic adenocarcinomas. The enforced expression of hK7 in BxPC-3 cells that express significant amounts of Dsg2 resulted in a marked increase in the shedding of soluble Dsg2, which is consistent with the notion that aberrant expression of hK7 in pancreatic tumors may result in diminished cell-cell adhesion and facilitate tumor cell invasion.
Figures

References
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Zetter BR. Adhesion molecules in tumor metastasis. Semin Cancer Biol. 1993;4:219–229. - PubMed
-
- Green KJ, Gaudry CA. Are desmosomes more than tethers for intermediate filaments? Nat Rev Mol Cell Biol. 2000;1:208–216. - PubMed
-
- Kowalczyk AP, Bornslaeger EA, Norvell SM, Palka HL, Green KJ. Desmosomes: intercellular adhesive junctions specialized for attachment of intermediate filaments. Int Rev Cytol. 1999;185:237–302. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

