Gemcitabine-based versus fluoropyrimidine-based chemotherapy with or without platinum in unresectable biliary tract cancer: a retrospective study
- PMID: 19091129
- PMCID: PMC2615782
- DOI: 10.1186/1471-2407-8-374
Gemcitabine-based versus fluoropyrimidine-based chemotherapy with or without platinum in unresectable biliary tract cancer: a retrospective study
Abstract
Background: There is no standard palliative chemotherapy regimen in biliary tract cancers (BTC). Fluoropyrimidine or gemcitabine, with or without platinum, are most frequently used. We conducted this study to clarify the efficacy of palliative chemotherapy in BTC.
Methods: Patients with unresectable BTC treated with palliative chemotherapy between Oct 2001 and Aug 2006 at Seoul National University Hospital were reviewed retrospectively. Histologically confirmed cases of intrahepatic cholangiocarcinoma, gallbladder cancer, extrahepatic bile duct cancer, and ampulla of Vater carcinoma were enrolled. We analyzed the efficacy of regimens: gemcitabine (G) versus fluoropyrimidine (F) and with or without platinum (P).
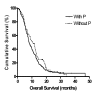
Results: A total of 243 patients were enrolled. 159 patients (65%) were male and the median age of the patients was 60 years (range 26-81). Intrahepatic cholangiocarcinoma, gallbladder cancer, extrahepatic bile duct cancer, and ampulla of Vater carcinoma were 92, 72, 58, and 21 cases, respectively. The median progression free survival (PFS) was 4.3 months (95% CI, 3.7-4.9) and median overall survival (OS) was 8.7 months (95% CI, 7.4-10.0). Ninety-nine patients received G-based chemotherapy (94 GP, 5 G alone), and 144 patients received F-based chemotherapy (83 FP, 61 F alone). The response rate (RR), disease control rate (DCR), PFS and OS of G-based chemotherapy versus F-based chemotherapy were 16.7% vs. 19.5% (P=0.591), 52.8% vs. 58.9% (P=0.372), 4.0 months vs. 4.3 months (P=0.816), and 7.8 months vs. 9.1 months (P=0.848), respectively. Sixty-six patients received F or G without P, and 177 patients received F or G with P. The RR, DCR, PFS and OS of chemotherapy without P versus chemotherapy including P were 12.7% vs. 20.6% (P=0.169), 46.0% vs. 60.6% (P=0.049), 3.3 months vs. 4.4 months (P=0.887), and 10.6 months vs. 8.1 months (P=0.257), respectively.
Conclusion: In unresectable BTC, F-based and G-based chemotherapy showed similar efficacy in terms of RR, DCR, PFS and OS. The benefit of adding P to F or G was not significant except for DCR. Further prospective studies which define the efficacy of various chemotherapeutic regimens in BTC are warranted.
Figures
References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA: a cancer journal for clinicians. 2007;57:43–66. - PubMed
-
- Glimelius B, Hoffman K, Sjoden PO, Jacobsson G, Sellstrom H, Enander LK, Linne T, Svensson C. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996;7:593–600. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources