Increased p38-MAPK is responsible for chemotherapy resistance in human gastric cancer cells
- PMID: 19091131
- PMCID: PMC2628930
- DOI: 10.1186/1471-2407-8-375
Increased p38-MAPK is responsible for chemotherapy resistance in human gastric cancer cells
Abstract
Background: Chemoresistance is one of the main obstacles to successful cancer therapy and is frequently associated with Multidrug resistance (MDR). Many different mechanisms have been suggested to explain the development of an MDR phenotype in cancer cells. One of the most studied mechanisms is the overexpression of P-glycoprotein (P-gp), which is a product of the MDR1 gene. Tumor cells often acquire the drug-resistance phenotype due to upregulation of the MDR1 gene. Overexpression of MDR1 gene has often been reported in primary gastric adenocarcinoma.
Methods: This study investigated the role of p38-MAPK signal pathway in vincristine-resistant SGC7901/VCR cells. P-gp and MDR1 RNA were detected by Western blot analysis and RT-PCR amplification. Mitgen-activated protein kinases and function of P-gp were demonstrated by Western blot and FACS Aria cytometer analysis. Ap-1 activity and cell apoptosis were detected by Dual-Luciferase Reporter Assay and annexin V-PI dual staining.
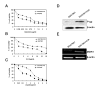
Results: The vincristine-resistant SGC7901/VCR cells with increased expression of the multidrug-resistance 1 (MDR1) gene were resistant to P-gp-related drug and P-gp-unrelated drugs. Constitutive increases of phosphorylated p38-MAPK and AP-1 activities were also found in the drug-resistant cells. Inhibition of p38-MAPK by SB202190 reduced activator protein-1 (AP-1) activity and MDR1 expression levels and increased the sensitivity of SGC7901/VCR cells to chemotherapy.
Conclusion: Activation of the p38-MAPK pathway might be responsible for the modulation of P-glycoprotein-mediated and P-glycoprotein-unmediated multidrug resistance in the SGC7901/VCR cell line.
Figures



Similar articles
-
SB203580, a specific inhibitor of p38-MAPK pathway, is a new reversal agent of P-glycoprotein-mediated multidrug resistance.Eur J Pharm Sci. 2001 Aug;14(1):29-36. doi: 10.1016/s0928-0987(01)00139-7. Eur J Pharm Sci. 2001. PMID: 11457647
-
Reversal of P-glycoprotein-mediated multidrug resistance in SGC7901/VCR cells by PPARgamma activation by troglitazone.J Huazhong Univ Sci Technolog Med Sci. 2010 Jun;30(3):326-31. doi: 10.1007/s11596-010-0351-6. Epub 2010 Jun 17. J Huazhong Univ Sci Technolog Med Sci. 2010. PMID: 20556576
-
AP-2α reverses vincristine-induced multidrug resistance of SGC7901 gastric cancer cells by inhibiting the Notch pathway.Apoptosis. 2017 Jul;22(7):933-941. doi: 10.1007/s10495-017-1379-x. Apoptosis. 2017. PMID: 28439677
-
Verotoxin-1 treatment or manipulation of its receptor globotriaosylceramide (gb3) for reversal of multidrug resistance to cancer chemotherapy.Toxins (Basel). 2010 Oct;2(10):2467-77. doi: 10.3390/toxins2102467. Epub 2010 Oct 25. Toxins (Basel). 2010. PMID: 22069561 Free PMC article. Review.
-
p38 Molecular Targeting for Next-Generation Multiple Myeloma Therapy.Cancers (Basel). 2024 Jan 6;16(2):256. doi: 10.3390/cancers16020256. Cancers (Basel). 2024. PMID: 38254747 Free PMC article. Review.
Cited by
-
Inhibition of p38 MAPK increases the sensitivity of 5-fluorouracil-resistant SW480 human colon cancer cells to noscapine.Oncol Lett. 2022 Feb;23(2):52. doi: 10.3892/ol.2021.13170. Epub 2021 Dec 17. Oncol Lett. 2022. PMID: 35035538 Free PMC article.
-
A20 and RBX1 Regulate Brentuximab Vedotin Sensitivity in Hodgkin Lymphoma Models.Clin Cancer Res. 2020 Aug 1;26(15):4093-4106. doi: 10.1158/1078-0432.CCR-19-4137. Epub 2020 Apr 16. Clin Cancer Res. 2020. PMID: 32299816 Free PMC article.
-
Epithelial-mesenchymal transition induced by GRO-α-CXCR2 promotes bladder cancer recurrence after intravesical chemotherapy.Oncotarget. 2017 Jul 11;8(28):45274-45285. doi: 10.18632/oncotarget.16786. Oncotarget. 2017. PMID: 28423359 Free PMC article.
-
CCDC134 is down-regulated in gastric cancer and its silencing promotes cell migration and invasion of GES-1 and AGS cells via the MAPK pathway.Mol Cell Biochem. 2013 Jan;372(1-2):1-8. doi: 10.1007/s11010-012-1418-4. Epub 2012 Aug 18. Mol Cell Biochem. 2013. PMID: 23070808
-
The human P-glycoprotein transporter enhances the type I interferon response to Listeria monocytogenes infection.Infect Immun. 2015 Jun;83(6):2358-68. doi: 10.1128/IAI.00380-15. Epub 2015 Mar 30. Infect Immun. 2015. PMID: 25824830 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

