C-peptide reverses TGF-beta1-induced changes in renal proximal tubular cells: implications for treatment of diabetic nephropathy
- PMID: 19091788
- PMCID: PMC2660188
- DOI: 10.1152/ajprenal.90500.2008
C-peptide reverses TGF-beta1-induced changes in renal proximal tubular cells: implications for treatment of diabetic nephropathy
Abstract
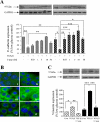
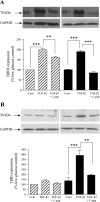
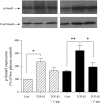
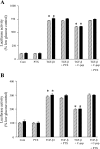
The crucial pathology underlying progressive chronic kidney disease in diabetes is tubulointerstitial fibrosis. Central to this process is epithelial-mesenchymal transformation (EMT) of proximal tubular epithelial cells driven by maladaptive transforming growth factor-beta1 (TGF-beta1) signaling. Novel signaling roles for C-peptide have recently been discovered with evidence emerging that C-peptide may mitigate microvascular complications of diabetes. We studied the potential for C-peptide to interrupt injurious TGF-beta1 signaling pathways and thus block development of EMT in HK2 human kidney proximal tubular cells. Cells were incubated with TGF-beta1 either alone or with C-peptide in low or high glucose. Changes in cell morphology, TGF-beta1 receptor expression, vimentin, E-cadherin, and phosphorylated Smads were assessed. Luciferase reporters were used to assess Smad activity. The cytoskeleton was visualized by TRITC-phalloidin staining. The typical TGF-beta1-stimulated, EMT-associated morphological alterations of proximal tubular cells, including increased vimentin expression, decreased E-cadherin expression, and cytoskeletal rearrangements, were prevented by C-peptide treatment. C-peptide also blocked TGF-beta1-induced upregulation of expression of both type I and type II TGF-beta1 receptors and attenuated TGF-beta1-mediated Smad phosphorylation and Smad transcriptional activity. These effects of C-peptide were inhibited by pertussis toxin. The results demonstrate that C-peptide almost completely reversed the morphological changes in PT cells induced by TGF-beta1 and suggest a role or C-peptide as a renoprotective agent in diabetic nephropathy.
Figures


References
-
- Al-Rasheed NM, Meakin F, Royal EL, Lewington AJ, Brown J, Willars GB, Brunskill NJ. Potent activation of multiple signalling pathways by C peptide in opossum kidney proximal tubular cells. Diabetologia 47: 987–997, 2004. - PubMed
-
- Al-Rasheed NM, Chana RS, Baines RJ, Willars GB, Brunskill NJ. Ligand-independent activation of peroxisome proliferator-activated receptor-gamma by insulin and C-peptide in kidney proximal tubular cells: dependent on phosphatidylinositol 3-kinase activity. J Biol Chem 279: 49747–49754, 2004. - PubMed
-
- Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem 275: 36803–36810, 2000. - PubMed
-
- Bland R, Walker EA, Hughes SV, Stewart PM, Hewison M. Constitutive expression of 25-hydroxyvitamin D3–1α-hydroxylase in a transformed human proximal tubule cell line: evidence for direct regulation of vitamin D metabolism by calcium. Endocrinology 140: 2027–2034, 1999. - PubMed
-
- Burns WC, Twigg SM, Forbes JM, Pete J, Tikellis C, Thallas-Bonke V, Thomas MC, Cooper ME, Kantharidis P. Connective tissue growth factor plays an important role in advanced glycation end product-induced tubular epithelial-to-mesenchymal transition: implications for diabetic renal disease. J Am Soc Nephrol 7: 2484–2494, 2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

