Xanthomonas campestris overcomes Arabidopsis stomatal innate immunity through a DSF cell-to-cell signal-regulated virulence factor
- PMID: 19091877
- PMCID: PMC2633826
- DOI: 10.1104/pp.108.126870
Xanthomonas campestris overcomes Arabidopsis stomatal innate immunity through a DSF cell-to-cell signal-regulated virulence factor
Abstract
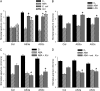
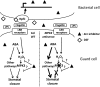
Pathogen-induced stomatal closure is part of the plant innate immune response. Phytopathogens using stomata as a way of entry into the leaf must avoid the stomatal response of the host. In this article, we describe a factor secreted by the bacterial phytopathogen Xanthomonas campestris pv campestris (Xcc) capable of interfering with stomatal closure induced by bacteria or abscisic acid (ABA). We found that living Xcc, as well as ethyl acetate extracts from Xcc culture supernatants, are capable of reverting stomatal closure induced by bacteria, lipopolysaccharide, or ABA. Xcc ethyl acetate extracts also complemented the infectivity of Pseudomonas syringae pv tomato (Pst) mutants deficient in the production of the coronatine toxin, which is required to overcome stomatal defense. By contrast, the rpfF and rpfC mutant strains of Xcc, which are unable to respectively synthesize or perceive a diffusible molecule involved in bacterial cell-to-cell signaling, were incapable of reverting stomatal closure, indicating that suppression of stomatal response by Xcc requires an intact rpf/diffusible signal factor system. In addition, we found that guard cell-specific Arabidopsis (Arabidopsis thaliana) Mitogen-Activated Protein Kinase3 (MPK3) antisense mutants were unresponsive to bacteria or lipopolysaccharide in promotion of stomatal closure, and also more sensitive to Pst coronatine-deficient mutants, showing that MPK3 is required for stomatal immune response. Additionally, we found that, unlike in wild-type Arabidopsis, ABA-induced stomatal closure in MPK3 antisense mutants is not affected by Xcc or by extracts from Xcc culture supernatants, suggesting that the Xcc factor might target some signaling component in the same pathway as MPK3.
Figures






References
-
- Allaway D, Schofield NA, Leonard ME, Gilardoni L, Finan TM, Poole PS (2001) Use of differential fluorescence induction and optical trapping to isolate environmentally induced genes. Environ Microbiol 3 397–406 - PubMed
-
- Allegre M, Daire X, Heloir MC, Trouvelot S, Mercier L, Adrian M, Pugin A (2007) Stomatal deregulation in Plasmopara viticola-infected grapevine leaves. New Phytol 173 832–840 - PubMed
-
- Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55 373–399 - PubMed
-
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415 977–983 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

