Temporal structure of receptor neuron input to the olfactory bulb imaged in behaving rats
- PMID: 19091924
- PMCID: PMC2657066
- DOI: 10.1152/jn.90902.2008
Temporal structure of receptor neuron input to the olfactory bulb imaged in behaving rats
Abstract
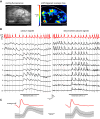
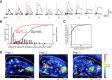
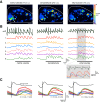
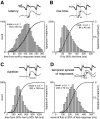
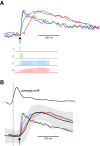
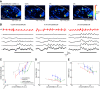
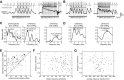
The dynamics of sensory input to the nervous system play a critical role in shaping higher-level processing. In the olfactory system, the dynamics of input from olfactory receptor neurons (ORNs) are poorly characterized and depend on multiple factors, including respiration-driven airflow through the nasal cavity, odorant sorption kinetics, receptor-ligand interactions between odorant and receptor, and the electrophysiological properties of ORNs. Here, we provide a detailed characterization of the temporal organization of ORN input to the mammalian olfactory bulb (OB) during natural respiration, using calcium imaging to monitor ORN input to the OB in awake, head-fixed rats expressing odor-guided behaviors. We report several key findings. First, across a population of homotypic ORNs, each inhalation of odorant evokes a burst of action potentials having a rise time of about 80 ms and a duration of about 100 ms. This rise time indicates a relatively slow, progressive increase in ORN activation as odorant flows through the nasal cavity. Second, the dynamics of ORN input differ among glomeruli and for different odorants and concentrations, but remain reliable across successive inhalations. Third, inhalation alone (in the absence of odorant) evokes ORN input to a significant fraction of OB glomeruli. Finally, high-frequency sniffing of odorant strongly reduces the temporal coupling between ORN inputs and the respiratory cycle. These results suggest that the dynamics of sensory input to the olfactory system may play a role in coding odor information and that, in the awake animal, strategies for processing odor information may change as a function of sampling behavior.
Figures
References
-
- Abraham NM, Spors H, Carleton A, Margrie TW, Kuner T, Schaefer AT. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron 44: 865–876, 2004. - PubMed
-
- Balu R, Larimer P, Strowbridge BW. Phasic stimuli evoke precisely timed spikes in intermittently discharging mitral cells. J Neurophysiol 92: 743–753, 2004. - PubMed
-
- Bathellier B, Buhl DL, Accolla R, Carleton A. Dynamic ensemble odor coding in the mammalian olfactory bulb: sensory information at different timescales. Neuron 57: 586–598, 2008. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

