Region-specific spike-frequency acceleration in layer 5 pyramidal neurons mediated by Kv1 subunits
- PMID: 19091962
- PMCID: PMC2677066
- DOI: 10.1523/JNEUROSCI.2940-08.2008
Region-specific spike-frequency acceleration in layer 5 pyramidal neurons mediated by Kv1 subunits
Abstract
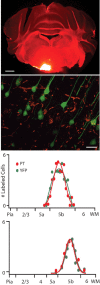
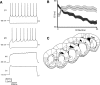
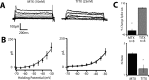
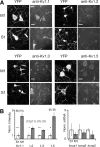
Separation of the cortical sheet into functionally distinct regions is a hallmark of neocortical organization. Cortical circuit function emerges from afferent and efferent connectivity, local connectivity within the cortical microcircuit, and the intrinsic membrane properties of neurons that comprise the circuit. While localization of functions to particular cortical areas can be partially accounted for by regional differences in both long range and local connectivity, it is unknown whether the intrinsic membrane properties of cortical cell types differ between cortical regions. Here we report the first example of a region-specific firing type in layer 5 pyramidal neurons, and show that the intrinsic membrane and integrative properties of a discrete subtype of layer 5 pyramidal neurons differ between primary motor and somatosensory cortices due to region- and cell-type-specific Kv1 subunit expression.
Figures


Similar articles
-
Intracellularly labeled pyramidal neurons in the cortical areas projecting to the spinal cord. I. Electrophysiological properties of pyramidal neurons.Neurosci Res. 2004 Dec;50(4):381-94. doi: 10.1016/j.neures.2004.08.006. Neurosci Res. 2004. PMID: 15567476
-
Functional roles of Kv1-mediated currents in genetically identified subtypes of pyramidal neurons in layer 5 of mouse somatosensory cortex.J Neurophysiol. 2018 Aug 1;120(2):394-408. doi: 10.1152/jn.00691.2017. Epub 2018 Apr 11. J Neurophysiol. 2018. PMID: 29641306 Free PMC article.
-
Corticospinal-specific HCN expression in mouse motor cortex: I(h)-dependent synaptic integration as a candidate microcircuit mechanism involved in motor control.J Neurophysiol. 2011 Nov;106(5):2216-31. doi: 10.1152/jn.00232.2011. Epub 2011 Jul 27. J Neurophysiol. 2011. PMID: 21795621 Free PMC article.
-
Functional roles of Kv1 channels in neocortical pyramidal neurons.J Neurophysiol. 2007 Mar;97(3):1931-40. doi: 10.1152/jn.00933.2006. Epub 2007 Jan 10. J Neurophysiol. 2007. PMID: 17215507
-
Somatic membrane potential and Kv1 channels control spike repolarization in cortical axon collaterals and presynaptic boutons.J Neurosci. 2011 Oct 26;31(43):15490-8. doi: 10.1523/JNEUROSCI.2752-11.2011. J Neurosci. 2011. PMID: 22031895 Free PMC article.
Cited by
-
Diversity of layer 5 projection neurons in the mouse motor cortex.Front Cell Neurosci. 2013 Oct 16;7:174. doi: 10.3389/fncel.2013.00174. eCollection 2013. Front Cell Neurosci. 2013. PMID: 24137110 Free PMC article.
-
Neuronal spike timing adaptation described with a fractional leaky integrate-and-fire model.PLoS Comput Biol. 2014 Mar 27;10(3):e1003526. doi: 10.1371/journal.pcbi.1003526. eCollection 2014 Mar. PLoS Comput Biol. 2014. PMID: 24675903 Free PMC article.
-
Evaluation of advances in cortical development using model systems.Dev Neurobiol. 2022 Jul;82(5):408-427. doi: 10.1002/dneu.22879. Epub 2022 May 29. Dev Neurobiol. 2022. PMID: 35644985 Free PMC article. Review.
-
Marked bias towards spontaneous synaptic inhibition distinguishes non-adapting from adapting layer 5 pyramidal neurons in the barrel cortex.Sci Rep. 2017 Nov 2;7(1):14959. doi: 10.1038/s41598-017-14971-z. Sci Rep. 2017. PMID: 29097689 Free PMC article.
-
Hierarchical connectivity and connection-specific dynamics in the corticospinal-corticostriatal microcircuit in mouse motor cortex.J Neurosci. 2012 Apr 4;32(14):4992-5001. doi: 10.1523/JNEUROSCI.4759-11.2012. J Neurosci. 2012. PMID: 22492054 Free PMC article.
References
-
- Adamson CL, Reid MA, Mo ZL, Bowne-English J, Davis RL. Firing features and potassium channel content of murine spiral ganglion neurons vary with cochlear location. J Comp Neurol. 2002;447:331–350. - PubMed
-
- Bekele-Arcuri Z, Matos MF, Manganas L, Strassle BW, Monaghan MM, Rhodes KJ, Trimmer JS. Generation and characterization of subtype-specific monoclonal antibodies to K+ channel alpha- and beta-subunit polypeptides. Neuropharmacology. 1996;35:851–865. - PubMed
-
- Benavides-Piccione R, Ballesteros-Yáñez I, DeFelipe J, Yuste R. Cortical area and species differences in dendritic spine morphology. J Neurocytol. 2002;31:337–346. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
