Organization and postembryonic development of glial cells in the adult central brain of Drosophila
- PMID: 19091965
- PMCID: PMC6671902
- DOI: 10.1523/JNEUROSCI.4844-08.2008
Organization and postembryonic development of glial cells in the adult central brain of Drosophila
Abstract
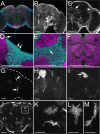
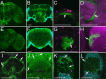
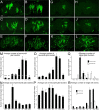
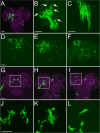
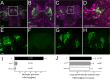
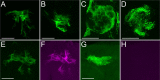
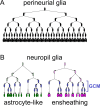
Glial cells exist throughout the nervous system, and play essential roles in various aspects of neural development and function. Distinct types of glia may govern diverse glial functions. To determine the roles of glia requires systematic characterization of glia diversity and development. In the adult Drosophila central brain, we identify five different types of glia based on its location, morphology, marker expression, and development. Perineurial and subperineurial glia reside in two separate single-cell layers on the brain surface, cortex glia form a glial mesh in the brain cortex where neuronal cell bodies reside, while ensheathing and astrocyte-like glia enwrap and infiltrate into neuropils, respectively. Clonal analysis reveals that distinct glial types derive from different precursors, and that most adult perineurial, ensheathing, and astrocyte-like glia are produced after embryogenesis. Notably, perineurial glial cells are made locally on the brain surface without the involvement of gcm (glial cell missing). In contrast, the widespread ensheathing and astrocyte-like glia derive from specific brain regions in a gcm-dependent manner. This study documents glia diversity in the adult fly brain and demonstrates involvement of different developmental programs in the derivation of distinct types of glia. It lays an essential foundation for studying glia development and function in the Drosophila brain.
Figures

References
-
- Awad TA, Truman JW. Postembryonic development of the midline glia in the CNS of Drosophila: proliferation, programmed cell death, and endocrine regulation. Dev Biol. 1997;187:283–297. - PubMed
-
- Awasaki T, Ito K. Engulfing action of glial cells is required for programmed axon pruning during Drosophila metamorphosis. Curr Biol. 2004;14:668–677. - PubMed
-
- Awasaki T, Tatsumi R, Takahashi K, Arai K, Nakanishi Y, Ueda R, Ito K. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron. 2006;50:855–867. - PubMed
-
- Bainton RJ, Tsai LT, Schwabe T, DeSalvo M, Gaul U, Heberlein U. moody encodes two GPCRs that regulate cocaine behaviors and blood-brain barrier permeability in Drosophila . Cell. 2005;123:145–156. - PubMed
-
- Beckervordersandforth RM, Rickert C, Altenhein B, Technau GM. Subtypes of glial cells in the Drosophila embryonic ventral nerve cord as related to lineage and gene expression. Mech Dev. 2008;125:542–557. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
