Prenatal to early postnatal nicotine exposure impairs central chemoreception and modifies breathing pattern in mouse neonates: a probable link to sudden infant death syndrome
- PMID: 19091979
- PMCID: PMC6671915
- DOI: 10.1523/JNEUROSCI.4441-08.2008
Prenatal to early postnatal nicotine exposure impairs central chemoreception and modifies breathing pattern in mouse neonates: a probable link to sudden infant death syndrome
Abstract
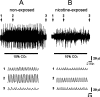
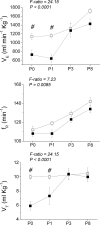
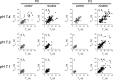
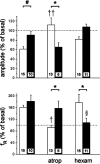
Nicotine is a neuroteratogen and is the likely link between maternal cigarette smoking during pregnancy and sudden infant death syndrome (SIDS). Osmotic minipumps were implanted in 5-7 d CF1 pregnant mice to deliver nicotine bitartrate (60 mg Kg(-1) day(-1)) or saline (control) solutions for up to 28 d. Prenatal to early postnatal nicotine exposure did not modify the number of newborns per litter or their postnatal growth; however, nicotine-exposed neonates hypoventilated and had reduced responses to hypercarbia (inhalation of air enriched with 10% CO(2) for 20 min) and hypoxia (inhalation of 100% N(2) for 20 s) at postnatal days 0-3 (P0-P3). In contrast, at postnatal day 8, nicotine-exposed neonates were indistinguishable from controls. Isolated brainstem-spinal cord preparations obtained from P0 to P3 nicotine-exposed neonates showed fictive respiration with respiratory cycles longer and more irregular than those of controls, as indicated by high short- and long-term variability in Poincaré plots. In addition, their responses to acidification were reduced, indicating compromise of central chemoreception. Furthermore, the cholinergic contribution to central chemosensory responses switched from muscarinic receptor to nicotinic receptor-based mechanisms. No significant astrogliosis was detectable in the ventral respiratory group of neurons with glial fibrillary acidic protein immunohistochemistry. These results indicate that nicotine exposure affects the respiratory rhythm pattern generator and causes a decline in central chemoreception during early postnatal life. Consequently, breathing would become highly vulnerable, failing to respond to chemosensory demands. Such impairment could be related to the ventilatory abnormalities observed in SIDS.
Figures



References
-
- Atluri P, Fleck MW, Shen Q, Mah SJ, Stadfelt D, Barnes W, Goderie SK, Temple S, Schneider AS. Functional nicotinic acetylcholine receptor expression in stem and progenitor cells of the early embryonic mouse cerebral cortex. Dev Biol. 2001;240:143–156. - PubMed
-
- Bamford OS, Carroll JL. Dynamic ventilatory responses in rats: normal development and effects of prenatal nicotine exposure. Respir Physiol. 1999;117:29–40. - PubMed
-
- Bamford OS, Schuen JN, Carroll JL. Effect of nicotine exposure on postnatal ventilatory responses to hypoxia and hypercapnia. Respir Physiol. 1996;106:1–11. - PubMed
-
- Benowitz NL, Jacob P., 3rd Daily intake of nicotine during cigarette smoking. Clin Pharmacol Ther. 1984;35:499–504. - PubMed
-
- Benowitz NL, Jacob P., 3rd Nicotine and cotinine elimination pharmacokinetics in smokers and nonsmokers. Clin Pharmacol Ther. 1993;53:316–323. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
